Product Overview
Compared to other EDC solutions on the market from MediData, DataTrack, Omnicomm and others, TrialStat is the most all-encompassing suite of study management tools that can be seamlessly connected to external data sources such as EMR, wearables, and other clinical and non-clinical data and information sources.
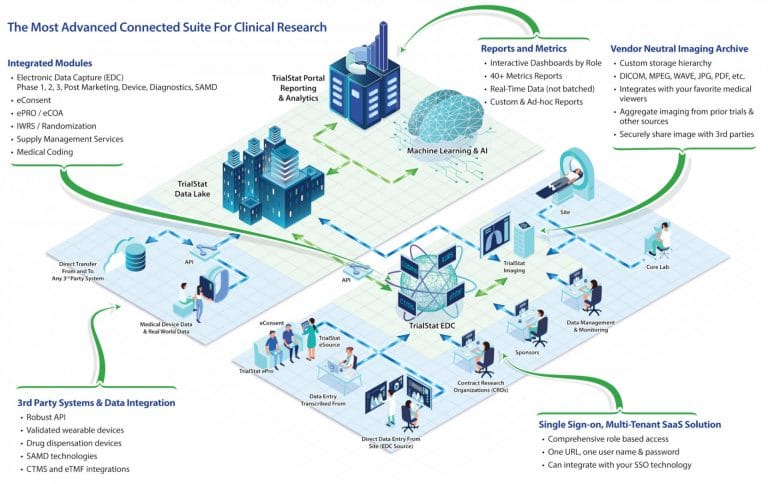
TrialStat offers a fully unified platform suitable for all phases and types of trials. Our single-sign-on, multi-tenant EDC suite includes modules for Randomization, Adjudication, Coding, Safety, Patient Diaries/ePRO, Vendor Neutral Imaging Archive, a robust Reporting and Analytics Portal and custom Machine Learning programs.
With a focus on data analytics, TrialStat offers real-time reporting, data extracts on demand, and analytics across a single study, a program, or your entire research portfolio – providing all stakeholders relevant, customized, timely insight into all aspects of study data and highlighting areas of risk or potential delays.
Highlights of TrialStat EDC
- Solution of choice for the successful execution of over 500 clinical trials.
- Of those trials, 96% of studies launched on time and database lock happened within 10 days on more than 92% studies.
- Built to meet the needs of users across all study phases including pilot / proof of concept studies, Phase I, II, III and IV studies, as well as medical device and diagnostics.
- Access from any browser, mobile device, tablet, laptop or desktop computer, without the requirements of any proprietary browser plugins or desktop software installs.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
- Price per Trial sites
More Information
A Fully Unified eClinical Suite with Premium Features and Capabilitie
- Completely Customizable eCRFs
- CDASH Compliant CRF Library
- Randomization / IWRS
- Inventory Management
- CDASH Compliant CRF LIbrary
- Integrate Real World Data
- Patient Reported Outcomes (ePRO)
- Browser Based & Mobile Responsive
- CTMS
- Comprehensive Edit Checks
- Dynamic Skip Logic & Comprehensive Edit Checks
- HTML 5 Complicant DICOM Viewer - CE Class IIa Certified
- Image Management
- 2-4 Week Build Time
- Configurable Study Workflow
- Real-Time Data Visualization
- Make Critical Decisions Sooner
- Flexible Data Capture
- Bar Code Integration
- Real-Time Monitoring, Reporting & Validation
- Integrated Real-Time Reporting
- Multi-Lingual Support
- Replicate Entire Studies
- 40+ Standard Reports
- Configurable Reports & Dashboards
- Drill Down Into Your Data With Interactive Reports
About the company
With 20 years of experience, global reach, and clients ranging from diagnostic start-ups to international pharmaceuticals, TrialStat is the right choice for your next study. At TrialStat, our technology solutions and services are developed and enhanced hand-in-hand with our users to uniquely address their needs and better support the life-changing work they do.
Whether designing and implementing world-class eClinical technology solutions for new studies, or providing data management and technology integration services in a rescue scenario, we’ve built our reputation through the execution of hundreds of successfully run studies, backed by our proven reliability and quick execution.