Product Overview
Agatha Clinical – Smarter eTMF for Faster, Compliant Trials
Agatha Clinical is a cloud-based electronic trial master file (eTMF) solution that connects all participants and processes in one unified platform. Built on the TMF Reference Model, it includes standard templates to help your team get started within hours or days—not weeks.
With configurable workflows, you can map and enforce best practices across the entire trial lifecycle. This means your team captures every action, connects all stakeholders, and maintains consistent documentation from start to finish.
COMPLETE
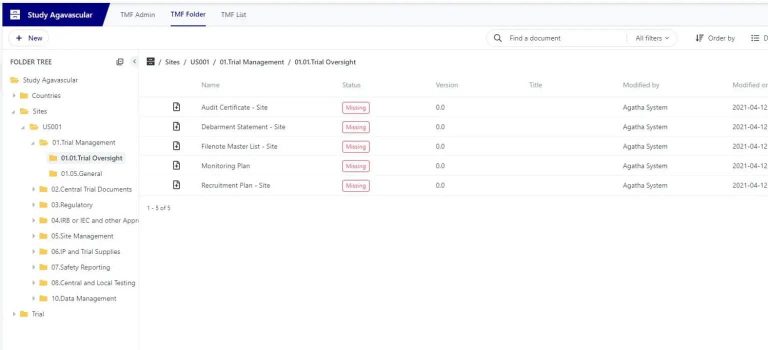
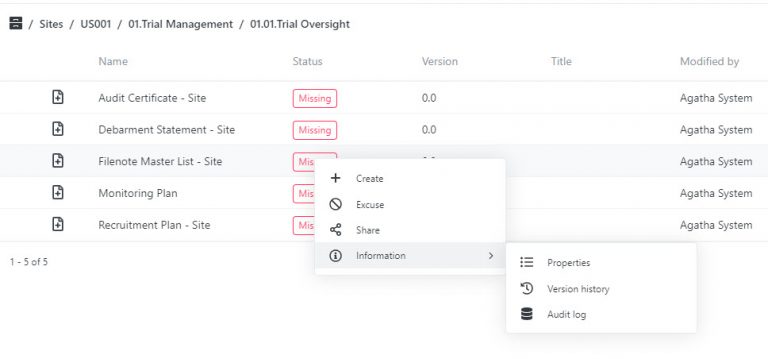
Make sure every essential document is present and accounted for. Agatha Clinical’s advanced placeholder technology highlights missing documents instantly, ensuring nothing slips through the cracks. Whether managing a single site or a global trial, you stay in full control and ready for inspection.
COMPLIANT
Agatha was designed to meet strict regulatory standards like 21 CFR Part 11. All applications run in a secure, high-performance cloud environment that is prevalidated for clinical use. This ensures data integrity and supports seamless regulatory compliance.
INSPECTION READY
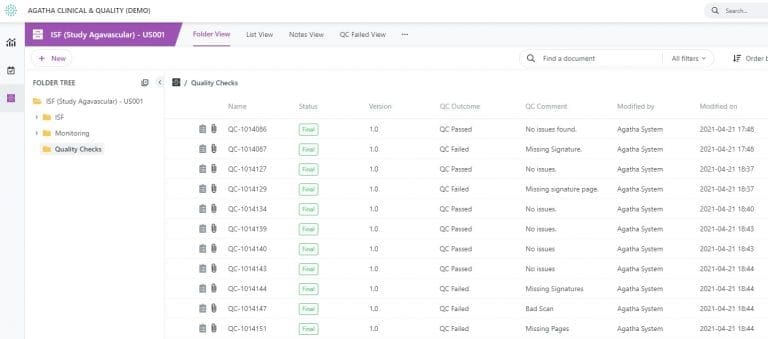
Prepare for audits with confidence. Agatha ensures all documents include critical elements such as digital signatures, audit trails, and version control. You can assign documents for quality checks and monitor review progress with customizable dashboards and views.
With Agatha Clinical, you get a complete, compliant, and inspection-ready eTMF solution that scales with your study needs.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Managing Clinical Trials is Complex – Use the right eTMF clinical trial software
Maintaining a TMF is a challenging activity – Make it easy with an eTMF
Clinical trials are complex projects. You are working with many participants, each with different responsibilities and working in different locations. That complexity is amplified by the process itself, which includes extensive document creation, review, and approval steps. You need a better strategy than using paper binders and processes managed through spreadsheets to maintain your clinical documents. You need an eTMF (electronic trial master file) application to guarantee document quality.
Create and Manage Sites & Clinical trial content
Forget paper documents: create and manage new TMF files based on the TMF Reference Model. Combine documents and forms to accelerate study processes.
Access Workspaces and Tasks in the Dashboard
View all clinical trials in the dashboard and quickly see and access tasks assigned to you. Use the configurable views to inspect, verify, and identify gaps in the expected eTMF content at any time.
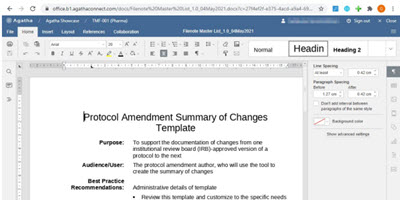
Author and Edit Documents Directly in the Electronic Trial Master File
Co-Author and edit Microsoft Word, Excel, and PowerPoint files directly in the application, without the need for Microsoft Office.
Ensure Quality Checks Are Completed
Designate documents for quality checks and track the quality review process using custom views.
Ensure compliance to requirements of regulatory agencies
eTMF software ensures your clinical trial documentation from all clinical trial stakeholders is compliant with regulatory requirements. Monitor that all essential documents include all required elements such as digital signatures and audit trails for inspectors.
About the company
Virtually all clinical and compliance solutions are based on traditional document management approaches. You know, check-in, check-out, yada yada. Agatha breaks the mold with better technologies to deliver a solution to manage end-to-end processes, not just documents. Agatha’s founders, saw firsthand that clinical and compliance solutions available in the market are built on excessively complex platforms and took far too long to bring to productive use. Lead times for implementing these unwieldy and costly systems are often as long as six months.
Clinical trials and compliance processes are the bridge between developing new drugs, devices, and therapies and getting them to the patients who desperately need them. Every day managing trials and compliance activities mean another day before a new drug or treatment is available to patients.
They set out to prove that a new class of cloud-first, ready-to-use applications could shorten the time required to get critical new drugs and treatments to patients.