Product Overview
PhlexTMF – Purpose-Built eTMF Software Backed by AI and TMF Expertise
PhlexTMF ensures your Trial Master File is complete, timely, and accurate throughout every stage of the trial and across the full document lifecycle. Unlike generic content platforms, PhlexTMF is 100% dedicated to TMF best practices and built specifically for life sciences.
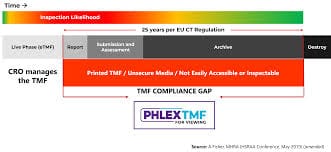
At the heart of PhlexTMF is the industry’s only pre-trained AI engine for TMF management. This powerful tool minimizes misfiles and metadata errors during the critical document upload stage, instantly improving TMF quality and lowering inspection risk.
Why Choose PhlexTMF?
- Built for TMF, Not Just Document Storage
Unlike eClinical platforms or repurposed systems, PhlexTMF was designed from the ground up by TMF experts to support regulatory compliance and operational efficiency. - Pre-Trained AI That Works from Day One
Trained on millions of TMF documents and guided by hundreds of TMF professionals, PhlexTMF’s AI suggests the correct classification and metadata right after upload, ensuring documents are accurate from the start. - Reduce Errors and Remediation Time
By preventing misfiles and metadata mistakes, PhlexTMF helps your team avoid costly rework and stay audit-ready.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
AI TIPS AT UPLOAD
Prevent document filing errors before they occur with suggestions based on millions of documents and the embedded guidance of TMF experts - improving TMF quality and reducing inspection risk.
SIMPLIFIED STUDY SETUP
Capture what is unique about a trial with study setup questions which are able to address specific trial parameters and document requirements, while still enforcing filing standards.
TMF EXPORTING
Easily transport your TMF between organizations and systems in the Exchange Mechanism format, ensuring order to the interchange of your TMF content, metadata, audit trail and e-signature information.
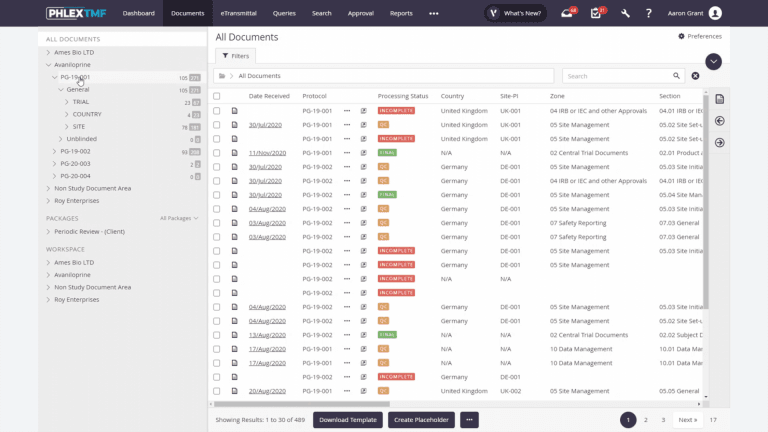
INTUITIVE USER INTERFACE
A simple interface with a unique combination of tree and search navigation which offers flexible document location options which are preferred by auditors and inspectors.
eTMF EVENT MANAGEMENT
Capture study changes such as protocol amendments that affect expected documents, plus track collection and quality control event stories to verify completion.
INTELLIGENT PLACEHOLDERS
Define where documents are expected which drives completeness metrics; simplify uploads by removing the need to fill in numerous fields for every document.
OVERSIGHT & REPORTING
User-configured dashboards dynamically filter results and can use heatmap visualization to show TMF timeliness and quality.
TMF QUALITY REVIEW
PhlexTMF Quality Review tools let you quickly and easily conduct regular, risk-based, or milestone-driven completeness reviews.
PROCESS MANAGEMENT
Flexible workflows help designate the path of a Trial Master File document from upload all the way through to inspection readiness.
eQUERY TRACKING SYSTEM
Identify, communicate and track remediation of Trial Master File issues, eliminating multiple communication paths and capturing all relevant information in one place.
WIZARD-DRIVEN COMPLETENESS
Easily and visually identify missing documents with views that show documents received vs expected in real-time across trial, country, site, zone, vendor, and more.
About the company
PharmaLex is now part of Cencora, a leading global pharmaceutical solutions organization centered on improving lives around the world.
PharmaLex adds to Cencora’s expanding suite of pharma solutions and serves the pharma, biotech, and medtech industries. We guide clients from early strategic planning activities and non-clinical requirements through clinical development, regulatory submission processes and post-approval / maintenance post-launch activities. Our experts use technology-elevated solutions to support clients through the entire product lifecycle.