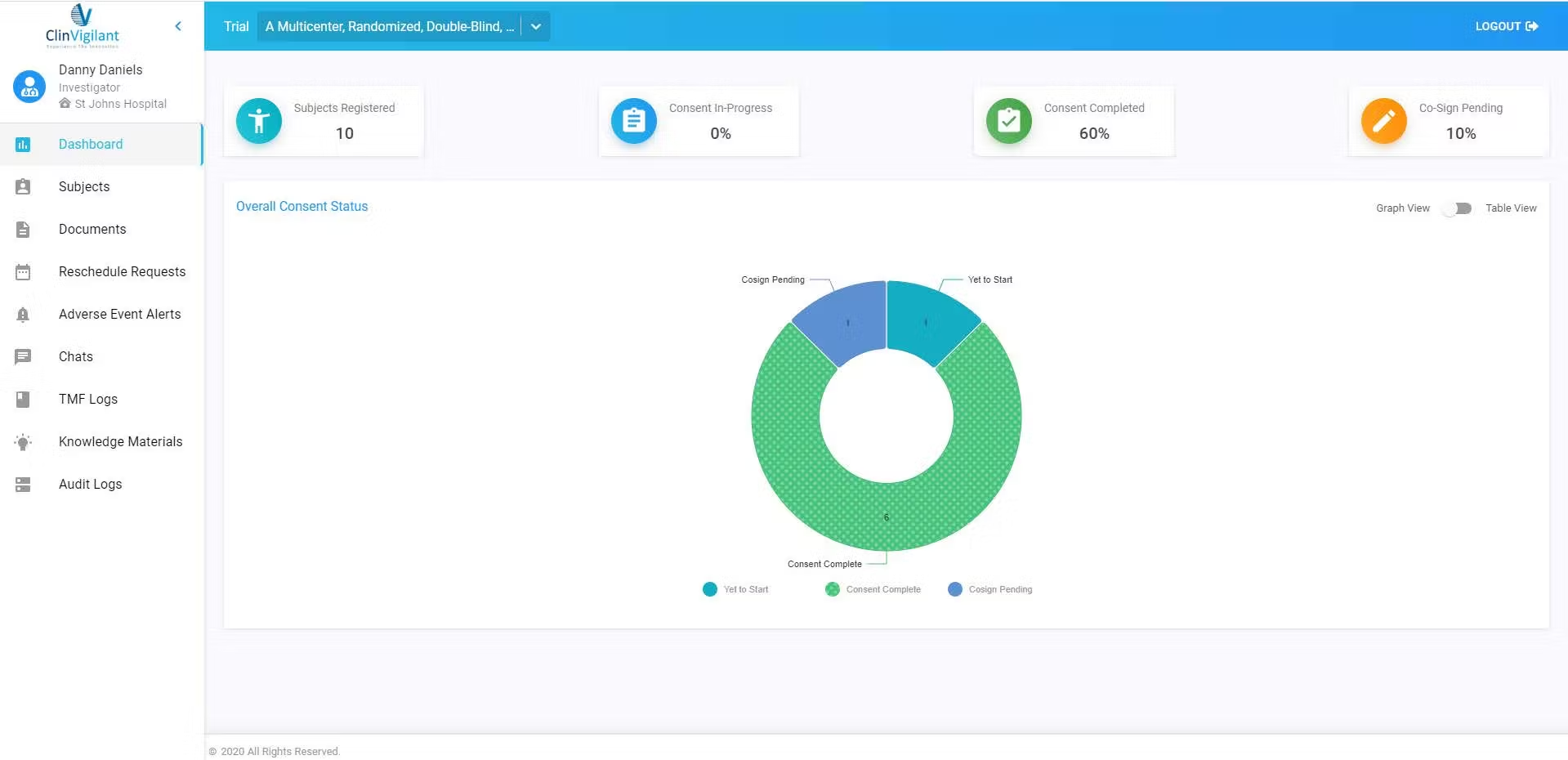
Product Overview
ClinVigilant CTMS is a next-generation solution that transforms how clinical trials are managed. It improves efficiency, oversight, and compliance, helping research teams stay ahead in today’s demanding clinical environment.
Built for modern trials, ClinVigilant CTMS offers powerful tools to streamline study management from start to finish. Trial managers, coordinators, and researchers can easily plan, execute, and track every phase of a clinical trial with confidence.
The system simplifies key processes like patient recruitment, data tracking, regulatory compliance, and financial management. With its user-friendly design and advanced features, it ensures that trials run smoothly and meet the highest standards.
ClinVigilant CTMS also enhances collaboration. It keeps all stakeholders connected and informed with a seamless flow of real-time information. Whether managing multiple sites, tracking enrollment, or generating timely reports, this platform delivers consistent and reliable support.
More than just software, ClinVigilant CTMS is a complete ecosystem that empowers teams to deliver better outcomes and shape the future of clinical research.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Trial Planning and Design
Helps in the planning and design phase of clinical trials, including defining objectives, methodologies, and participant criteria.
Site Management and Monitoring
Facilitates the management of multiple trial sites, including tracking site visits, site reports, follow-up activities, site performance, managing site payments, and ensuring compliance with trial protocols.
Participant Recruitment and Management
Streamlines participant recruitment processes and manages participant data, schedules, and communications throughout the trial.
Document Management
Provides a centralized repository for all trial-related documents, ensuring easy access, version control, and compliance with regulatory standards.
Regulatory Compliance
Helps ensure compliance with local and international regulatory requirements, including FDA regulations, GCP standards, and GDPR.
Budgeting and Financial Management
Assists in the creation and tracking of trial budgets, managing financial transactions, and ensuring financial compliance and reporting.
Data Management and Integration
Integrates with Electronic Data Capture (EDC) systems and other data sources for efficient data management and analysis.
Reporting and Analytics
Offers robust reporting and analytics tools to generate insights on trial progress, site performance, participant enrollment, and other key metrics.
Scheduling and Visit Tracking
Manages schedules for trial-related activities, including participant visits, monitoring visits, and key milestones.
Safety and Adverse Event Management
Facilitates the tracking and reporting of adverse events and ensures timely communication with regulatory bodies.
Vendor and Stakeholder Management
Manage contract and interactions with vendors, sponsors, and other stakeholders, facilitating communication and collaboration.
Customization and Scalability
Can be customized to suit the specific needs of different trials and is scalable to accommodate trials of varying sizes and complexities.
Remote Access and Mobile Compatibility
Provides remote access and mobile compatibility, allowing trial staff and participants to access the system from anywhere.
Security and Data Protection
Ensures the security of sensitive trial data with robust data protection measures and compliance with data privacy laws.
User Training and Support
Includes comprehensive user training and support services to ensure effective use of the software.
About the company
From decentralized trials to our clinical trial consulting services, our therapeutic, technical and functional team is underprop by the culture of ethics and patient safety. Our strong conviction and commitment is to our clients in bringing the next generation quality therapies faster to the market.
Our experts and developers can work on complex business challenges with your team to help you achieve your goals and define a roadmap of necessary changes, whether by implementing new solutions or transforming existing legacy processes