Product Overview
RegDocs365™ – The Smarter Way to Manage Life Science Documents
Are you struggling to manage critical documentation efficiently? RegDocs365™ is built for life science organizations of all sizes. It combines a compliant foundation with industry-specific features to simplify and streamline content management.
Organized and Ready to Use
RegDocs365™ offers preconfigured folder structures for departments like R&D, finance, and legal. This ensures your documents meet regulatory standards and stay organized and easy to find.
Built for TMF and EDM
Whether you’re running clinical trials or preparing FDA submissions, RegDocs365™ supports Trial Master File (TMF) creation and Electronic Document Management (EDM), including eCTD formats. It helps you stay compliant at every step.
Powered by AI
Advanced AI features enhance your workflow. These include auto-classification, smart search, support for mergers and acquisitions, and smooth integration with legacy systems for easier data migration.
Compliant and Secure
RegDocs365™ complies fully with 21 CFR Part 11. It includes audit logging, strong security controls, and tools to collect regulated content from clinical sites and external partners.
Collaboration with Control
With granular access settings, you can share specific content externally without compromising control. Built-in security ensures your sensitive documents remain protected at all times.
Scalable and Flexible
As your needs grow, RegDocs365™ grows with you. Developed by Court Square Group, the platform integrates with consulting partners to add modules for quality management, CMC support, and more—tailored to your sector.
Deployment
- Web based
- Cloud Hosted
Pricing
- License Fee
- Price per Trial sites
More Information
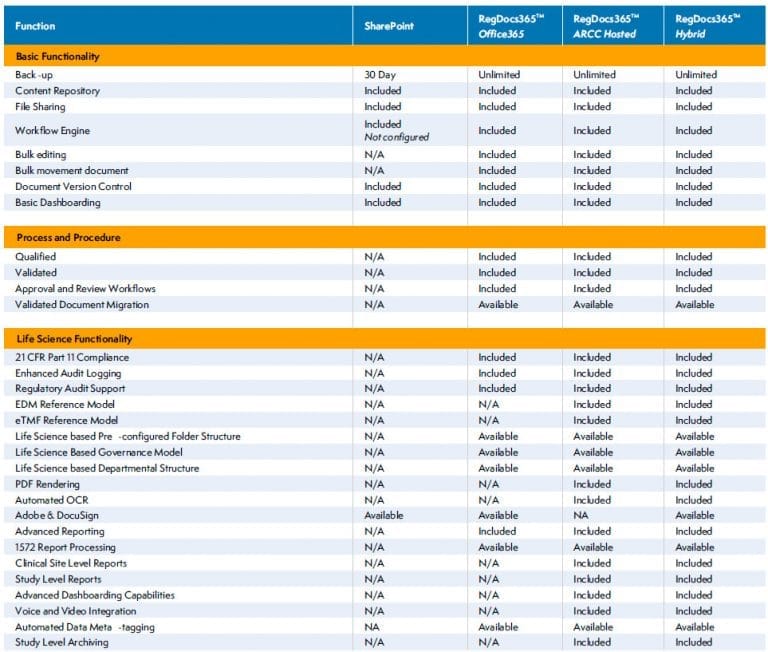
Basic Capabilities
- Qualified Microsoft based infrastructure including SharePoint and SQL Server
- Content management system preconfigured to EDM Reference Model for regulatory submissions and Electronic Trial Master File (eTMF)
- Submission-ready PDF renditions
- Collaborative review and approval workflows
Advanced Capabilities
- Configured for Trial MasterFile Reference Model (eTMF)
- Submission management for IND, MDF, ANDA, NDS, BLA Submissions
- Optional submission templates for eCTD document authoring
- Optional eCTD publishing services and software
About the company
RegDocs365 came about as a result of the collaboration between Court Square Group hosting a submission processing environment for eSubmissionSolutions. The founders, Keith Parent from Court Square Group and Antoinette Azevedo from eSubmissionSolutions had been working together for a number of years. Recognizing the significance of the environment that Antoinette was using for her client base we decided that this would be a great offering for small to mid-size companies looking to do their own Regulatory filings. By replicating the environment for other Regulatory customers we were able to have our first regulatory offerings featuring the DIA EDM and eTMF reference model.