Product Overview
Castor’s modular eConsent solutions improve clinical trial efficiency—from recruitment and screening to direct data capture and analysis. You can use each virtual component on its own or combine them into a complete eConsent platform for traditional, hybrid, or decentralized trials.
Boost patient access and diversity through remote enrollment. Use a custom recruitment portal and pre-screen participants with questionnaires to quickly identify qualified candidates. Once a participant consents, the system automatically updates their data in Castor EDC, preparing it for the next phase.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
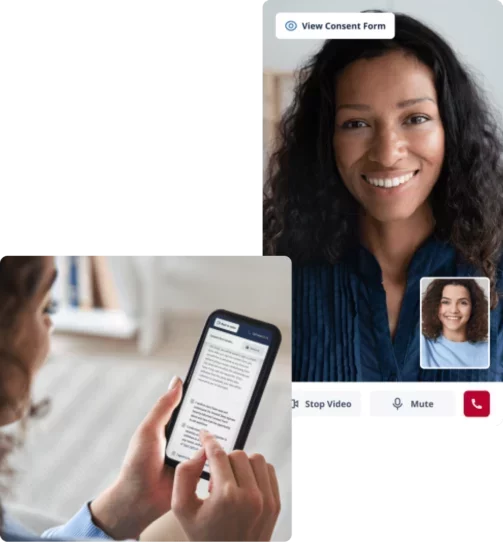
Consent study participants remotely with secure video visits
Host remote consent visits with video and engage participants in the comfort of their home. No switching between applications – video calls, signatures, and questionnaires are all built-in to reduce complexity and increase your patient’s experience.
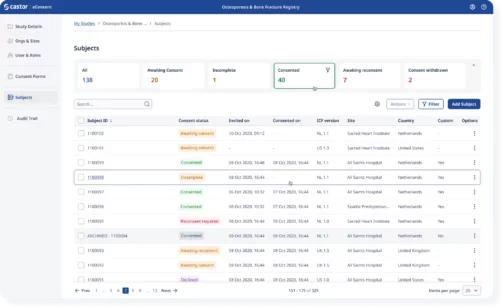
Easily track enrollment progress and gain real-time insights
Study coordinators can see in real-time where patients are in their enrollment process and assist patients while meeting regulatory requirements.
About the company
In 2012 Dr. Arts, our founder, invented Castor which enables researchers to easily capture and integrate high-quality data from any source on one compliant platform. Thousands of medical device, biotech, and academic researchers around the world are now using Castor’s eClinical solutions to accelerate their studies.
Castor currently employs more than 100 people that are passionate about transforming medical research across its headquarters in Amsterdam, the United States and remotely.