Product Overview
Ennov’s Pharmacovigilance suite simplifies the collection, management, assessment, and reporting of human or veterinary adverse events. It offers a single, unified database and includes advanced tools for signal detection and PV data analysis.
Our software is trusted by pharmaceutical, biological, and device companies, as well as contract manufacturers, CROs, and global health authorities.
A single authoritative source:
Track and manage all pharmacovigilance data in one place to streamline operations and ensure compliance.
Improved performance:
Replace manual, paper-based processes with automation. Boost productivity through automated PV case intake.
Ensure compliance:
Meet all regulatory requirements with confidence, including electronic reporting.
Increased visibility:
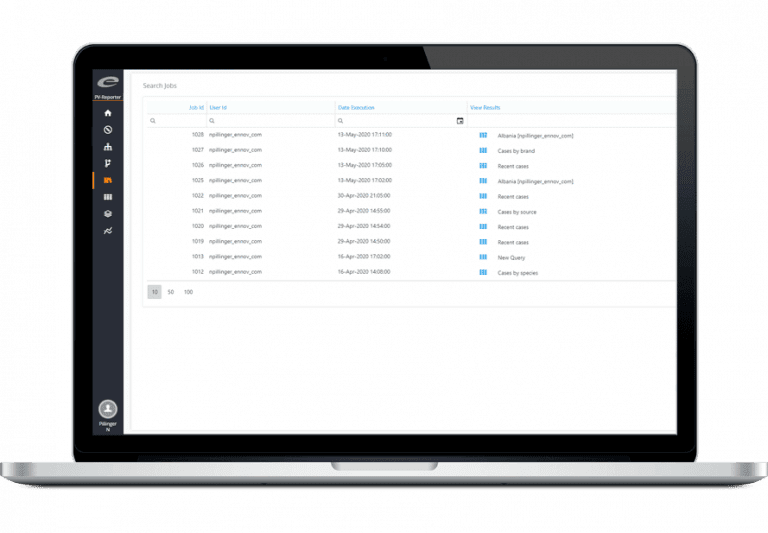
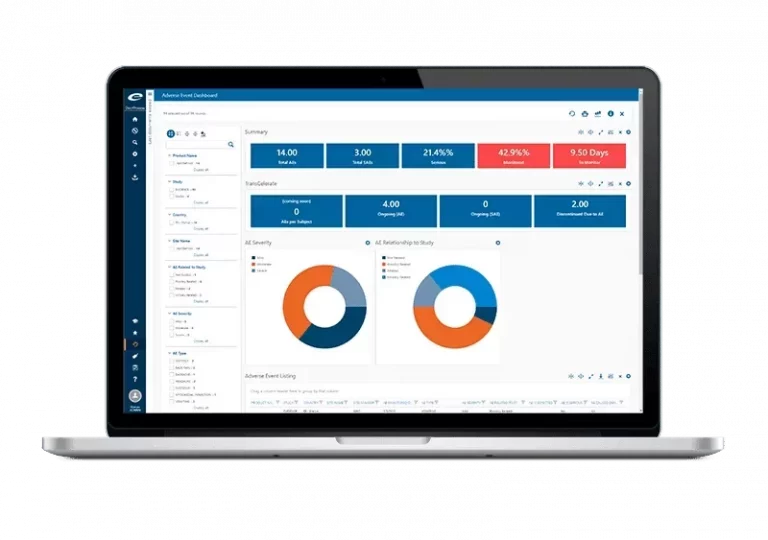
Access real-time insights into your safety database using dynamic dashboards and metrics.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Case Intake, Triage, Reporting
Animal and Human Health Pharmacovigilance Software
Our flexible software system is designed to support pharmacovigilance business processes and technical services case handling practices. It meets the associated company safety and worldwide regulatory reporting requirements. Used by pharmaceutical companies, CROs, and Pharmacovigilance outsourcing service providers, the software can record, report, and analyze clinical trial and post-marketing adverse events. It ensures the efficient and accurate intake of PV cases. It also seamlessly submits safety data to the FDA, EMA, or other regulatory authorities worldwide. This is achieved via the supplied E2B compliant gateway. Make controlled assessments and apply standardized coding, all while maintaining complete regulatory compliance. All common single case and aggregate reporting formats are accessible.
About the company
Headquartered in Paris, with offices in the US, UK, and Asia, Ennov provides the most original, comprehensive and cost-effective suite of software solutions for the Life Sciences industry. From leading pharmaceutical companies to emerging biotechs, we proudly serve over 250 companies and 250,000 users around the world.
For more than 20 years, we have been developing innovative, powerful and easy-to-use software for regulated content, data and process management. Our solutions are designed and built to support the entire Life Sciences R&D continuum including Clinical, Regulatory, Quality, Pharmacovigilance and Commercial. Ennov is ISO9001:2015 certified for all software products and processes and we boast a 100% success rate in customer audits.
Our solutions enjoy extremely high user adoption rates, thanks to our intuitive user interfaces and commitment to building solutions that work the way our users work. We also have very high customer satisfaction ratings with an on-time project delivery rate of 98.5% and an annual maintenance renewal rate of 96%.