Product Overview
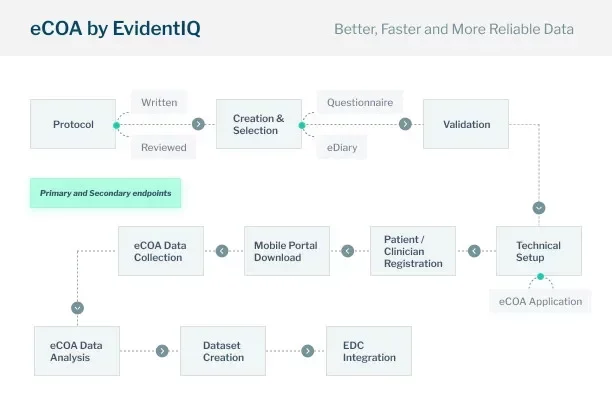
Our customers can choose our comprehensive end-to-end eCOA package or select specific components that meet their unique needs. The solution works independently of other eClinical technologies, including EDC systems. However, it can also integrate smoothly into any existing eClinical software landscape. It’s designed to capture reported outcome data in a compliant, efficient, and streamlined way.
Ensure success with robust, flexible technology
We support a bring-your-own-device (BYOD) approach across all devices. Our team helps customers review study protocols and recommend standard questionnaires or eDiaries tailored to primary and secondary endpoints. If needed, we build fully customized solutions. We validate all instruments against industry standards and medical requirements before deploying them in our eCOA application.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Highly configurable
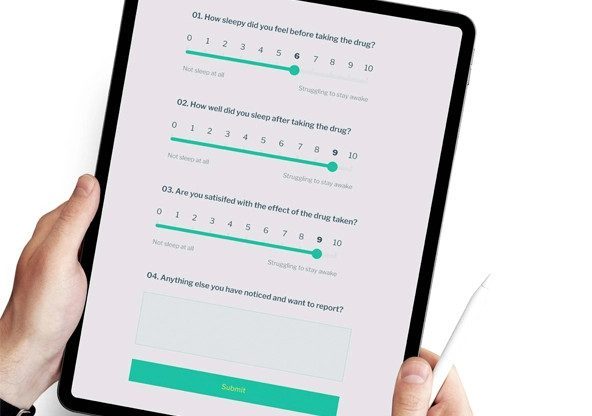
Build complex electronic data capture forms for use during in clinic or virtual visits. EvidentIQ eCOA data capture forms include all standard form fields, text, and scale based- questions, image hotspots, smart notifications, and data checks required for most protocols.
Ensure data integrity/quality
eCOA enables real-time data streams and accurate timestamping and data entries, which improve the quality of the data coming in for a given study. In addition, this data is encrypted and protected, ensuring patient data integrity.
About the company
Leveraging more than 50 years of combined experience providing innovative and creative ways to conduct clinical trials. End-to-end eClinical Solution
Meeting increasing customer demand across clinical operations and clinical data management needs with a suite of applications within a single integrated cloud platform, including EDC, CTMS, eTMF, eCOA, coding, remote monitoring, RBM, RTSM, Payments and eFeasibility. Geographical Reach
In-country patient communities platform in North America and Western Europe (EU5) and ability to conduct studies at a broader scale (LatAM, Asia…). Regulatory expertise to support broader geographical studies.