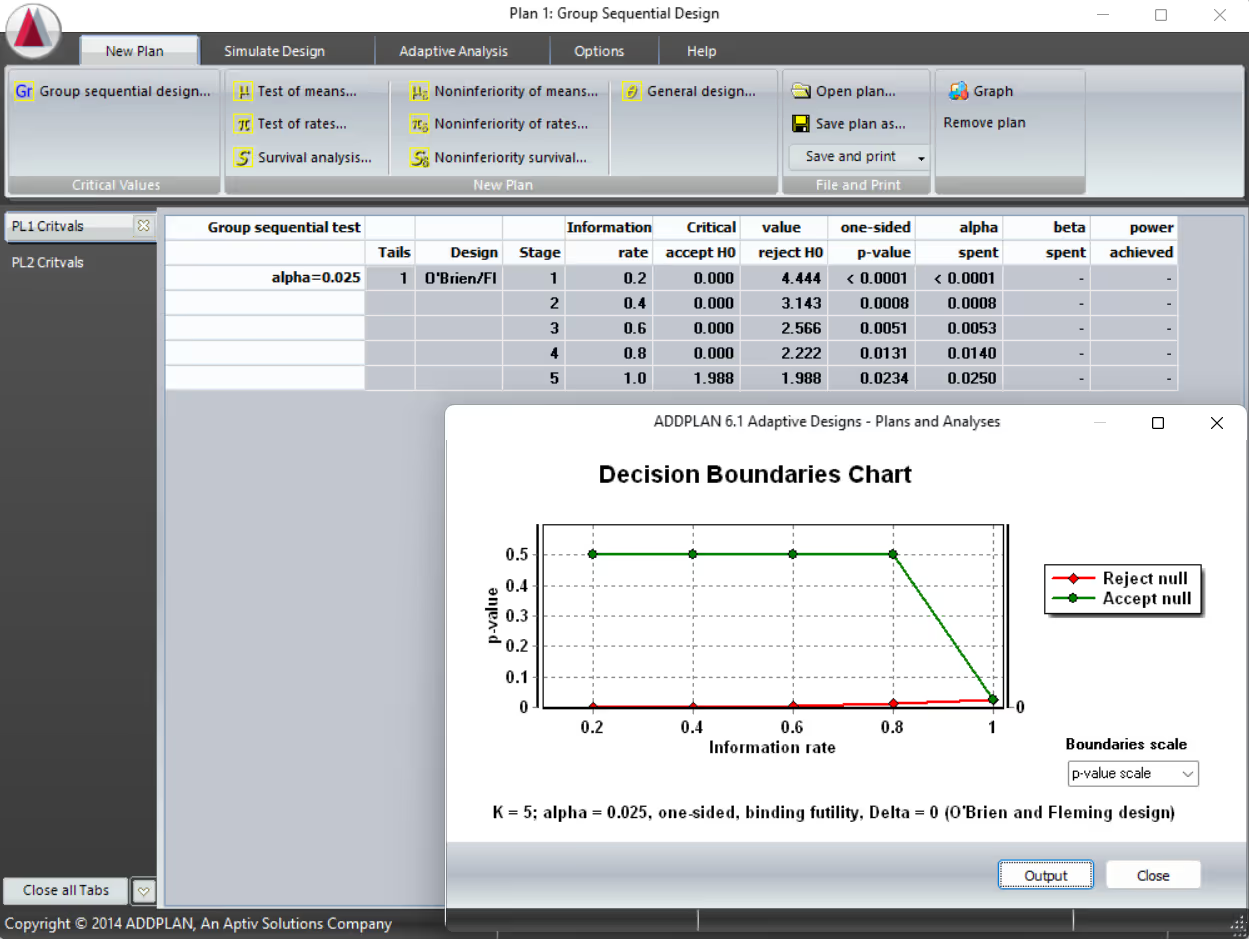
Product Overview
ADDPLAN (ADaptive Design PLANner) is trusted software for planning many types of adaptive clinical trials. Spanning Phases 1 to 4, it supports Multiple Comparison Procedures (MCP), Population Enrichment, and Dose Finding (MCP-Mod). Used by regulatory agencies, top pharma, and researchers, ADDPLAN enables smarter, faster, and more precise trial designs with a user-friendly interface.
Innovation made accessible: Run thousands of simulations in minutes to refine trial designs, understand uncertainty, and make data-driven decisions with confidence.
Built for every trial phase: Plan designs from early phase dose escalation and MCP-Mod to submission ready analytically controlled Multi-Arm Multi-Stage (MAMS) designs.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Comprehensive Trial Design Capabilities
ADDPLAN supports a wide range of adaptive clinical trial designs, providing flexibility and precision across every stage of development. Key design capabilities include:
- Dose Escalation: Compare a fixed rule mTPI escalation trial to a Bayesian logistic regression driven trial for finding the maximum tolerated dose.
- MCP-Mod: Use the Multiple Comparison Procedure and Modeling approach in a dose ranging study to find the best dose to use in a Phase 3 trial.
- Sample Size Re-estimation: Use conditional power to expand the maximum sample size of a trial if results are promising but not conclusive.
- Population Enrichment: Adaptively enrich the enrolling trial population to target the subjects that the treatment helps the most.
- MAMS Trials: Design and compare type I error controlled multiple arm, multiple stage trials in seconds.
- Surrogate endpoints: Use surrogate endpoints to select the best treatments or populations that benefit most when modeling survival data.
These design options allow development teams to explore, compare, and refine trial strategies to ensure maximum efficiency and success.
About the company
Berry Consultants (“Berry”) is a scientific consulting company specializing in innovative clinical trial design, Bayesian analysis, adaptive trial implementation, and software solutions for the pharmaceutical and medical device industry.
Berry was founded in 2000 by Donald Berry, PhD and his son, current President, Scott Berry, PhD. Since then, Berry has designed thousands of innovative adaptive trials for medical device, biotech, and pharmaceutical companies. In addition to Don and Scott, Berry currently employs full-time statistical and medical scientists to precisely tailor innovative flexible designs for each client.