Product Overview
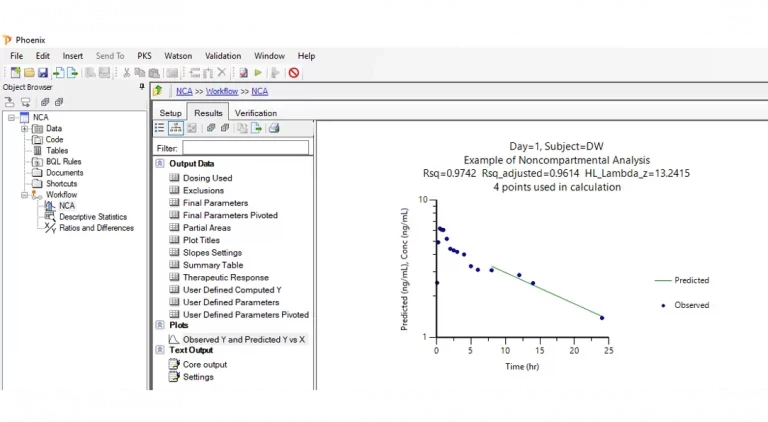
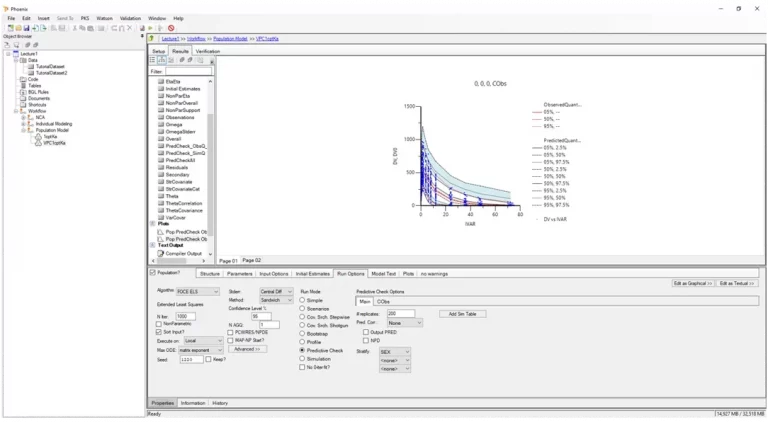
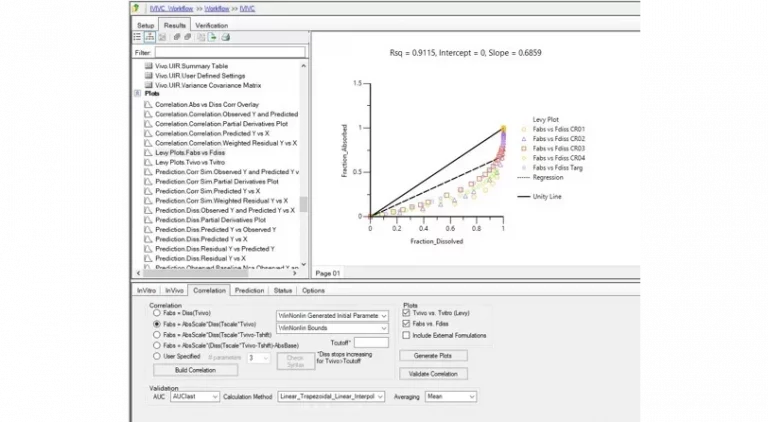
Phoenix™ delivers comprehensive tools for PK/PD modeling, empowering preclinical scientists and clinical pharmacologists to make critical drug development decisions like these with confidence, even for the most complex modalities.
Certara Phoenix bridges the gap between discovery and clinical phases by enabling robust analysis of pharmacokinetic (PK) and pharmacodynamic (PD) data in every application. From early preclinical evaluations to late stage regulatory submissions, Phoenix ensures precision, scalability, and compliance, supporting decision making across the drug development spectrum.
Certara holds ISO 27001 certification for Certara’s Information Security Management System (ISMS). We have implemented robust security controls, undergone rigorous risk assessments, and continuously strive for improvement. Phoenix ensures full compliance with global data protection standards, offering peace of mind for sensitive analysis.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Versatility for the PK/PD Scientist
Certara Phoenix bridges the gap between discovery and clinical phases by enabling robust analysis of pharmacokinetic (PK) and pharmacodynamic (PD) data in every application. From early preclinical evaluations to late stage regulatory submissions, Phoenix ensures precision, scalability, and compliance, supporting decision making across the drug development spectrum.
About the company
We transform drug development for the benefit of humans. Our commitment is to empower innovators, researchers, and regulatory experts with software and service solutions tailored exactly to meet their needs, fostering success across the entire drug development lifecycle.
Certara is dedicated to transforming drug discovery and development for good. We harness the power of biosimulation, advanced analytics, scientific, strategic and regulatory expertise to create a future where treatments reach patients faster and more efficiently.