Product Overview
As the global leader in patient-centric data analytics, Phesi can help you design better protocols from the outset and avoid costly amendments across your clinical development portfolio, resulting in shorter cycle times and faster cures
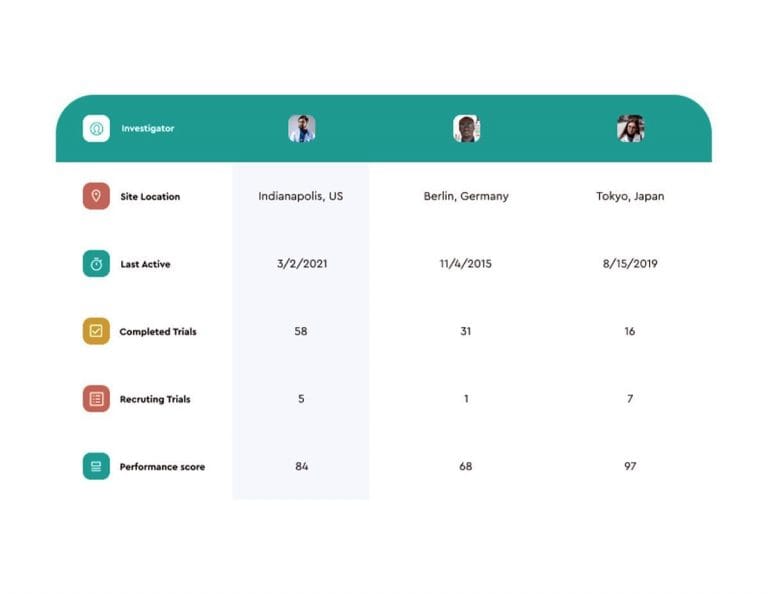
Patients are at the heart of our protocol design optimisation services. With the world’s largest clinical development database and our proven integrated approach to clinical data science, we provide insight from 70 million patients to inform and improve the success of your trials.
Let us help you target the right patients and make fast and accurate decisions, resulting in:
- Cost reduction and faster trial timelines
- Reduced patient burden
- Fewer protocol amendments
- A greater understanding of the competitor landscape
- Accurate enrollment cycle time predications
- Fewer non-performing sites
- Simplified ‘what if’ analysis with our predictive modelling
- Development of Digital Twins and Digital Control Arms
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
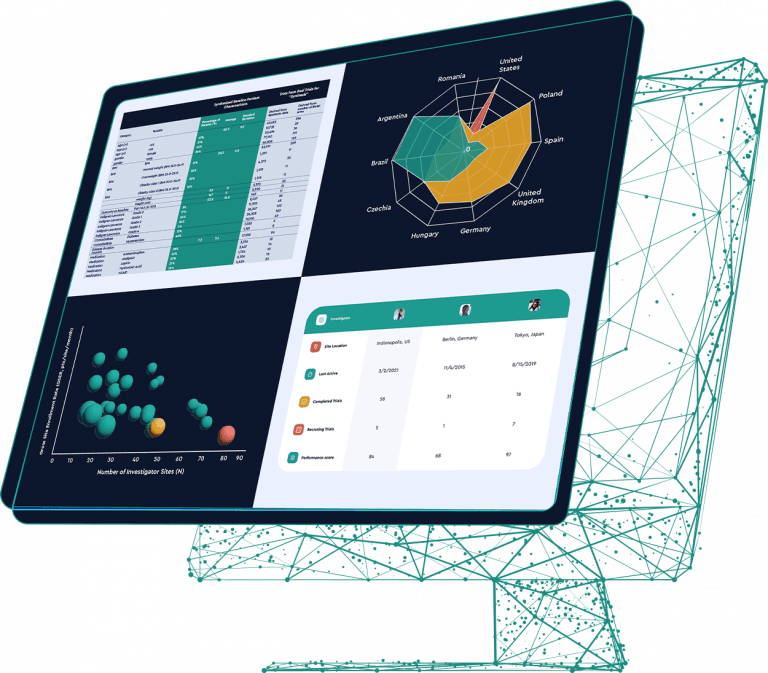
Phesi’s AI-powered Trial Accelerator™ advanced clinical data analytics platform collects and analyzes live data from more than 4,000 diseases. It includes trial and site information from 485,000+ trials, including more than 600,000 principal investigators in nearly 200 countries. By applying our proprietary integrated data science approach and advanced protocol design analytics to this data, we drive successful clinical development and commercialization.
Planning a clinical study means answering a lot of questions. Phesi’s access to such a volume of data removes guesswork. Allowing you to make decisions based wholly on insights from data, confidently and quickly. Including:
About the company
Phesi provides comprehensive clinical development, analytical products and services for biopharmaceutical companies around the world. Our integrated offerings cover the entire clinical development process – from development planning and indication assessment to protocol evaluation, site selection, and trial implementation management.