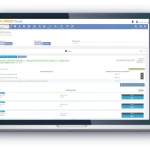
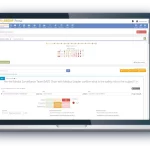
Product Overview
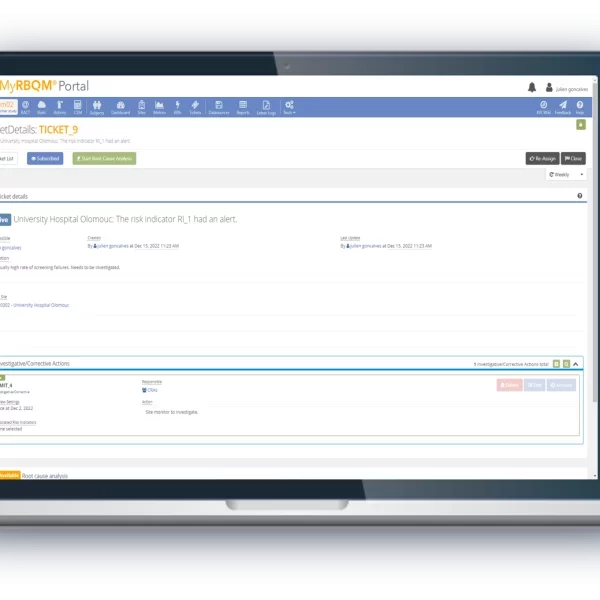
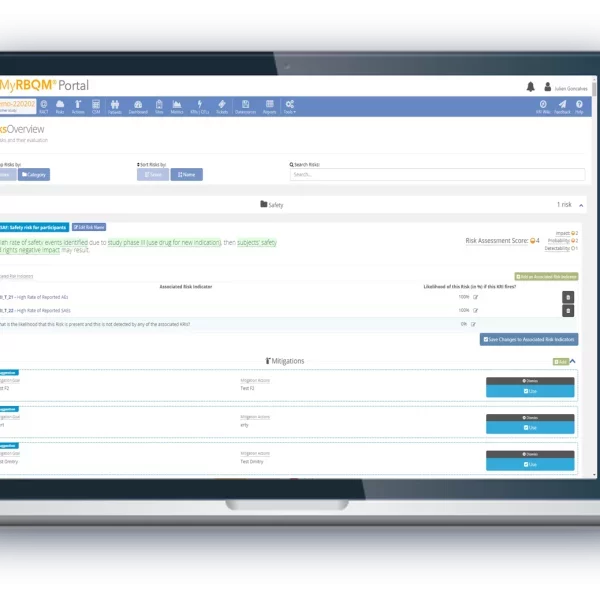
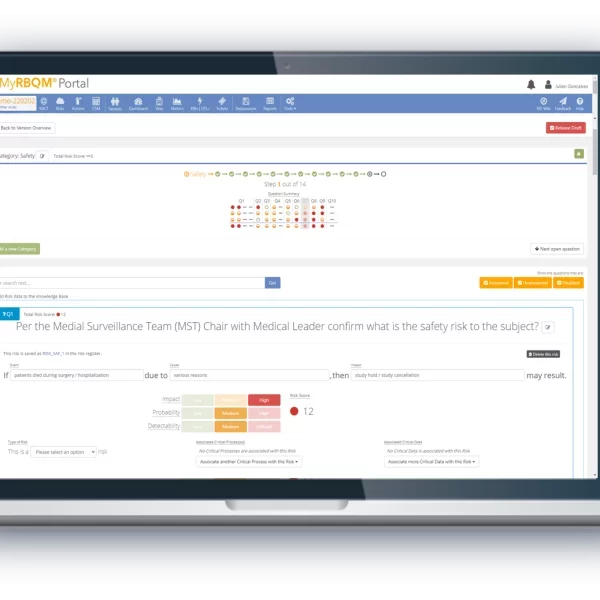
From study start-up to close out, the Cyntegrity RBQM Portal allows hands-on risk management across all data levels, complemented with ML-driven predictive analytics. With role-based access control, admins can control who needs access to what data, providing peace-of-mind that each stage of your operations remains focused and efficient.
- Reduce the need for 100% Source Data Verification.
- End-to-end traceability and data transparency, with time-stamped audit trails.
- Collectively identify and address issues before they occur.
- Improve monitoring efficiency from start to finish.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
The ICH E6, E8 and Q9 guidelines encourage users to embrace a quality-by-design and risk-based approach to clinical trials, in place of the old-school "one size fits all" approach. Regulatory bodies too are beginning to use risk-based approaches to understand where issues may lie within new drug approval submissions. By using the MyRBQM Portal, you can rest assured that your data will be in line with regulatory requirements and will always be audit-ready.
- Audit-readiness at all times with transparent, time-stamped and readable audit reports.
- Data transparency and traceability ensures that auditors have full trust in the data presented.
- Identify which studies, sites or regions have issues before triggering or receiving audit visits.
- Actionable GCP-compliant workflow with integrated Critical Processes and Data.
About the company
Cyntegrity is an expert team of RBQM pioneers who want nothing more than satisfied clients and rewarding projects. Our collective focus on risk-based quality management has led us to prioritize regulatory compliance, where patient safety and data quality are central, over finding ways to reduce its cost.
The best way to mitigate conflicts of interest is to avoid them in the first place. Cyntegrity is a holistic RBQM solution provider — not a Sponsor, CRO or e.g. EDC provider — so we can be objective about data quality audits. Our independent specialists supported by our compliant technology and processes bring all risks and stakeholders in scope, from start to finish.