Product Overview
Creating and maintaining regulatory submissions that are compliant with regulatory standards around the globe is complex and time-consuming.
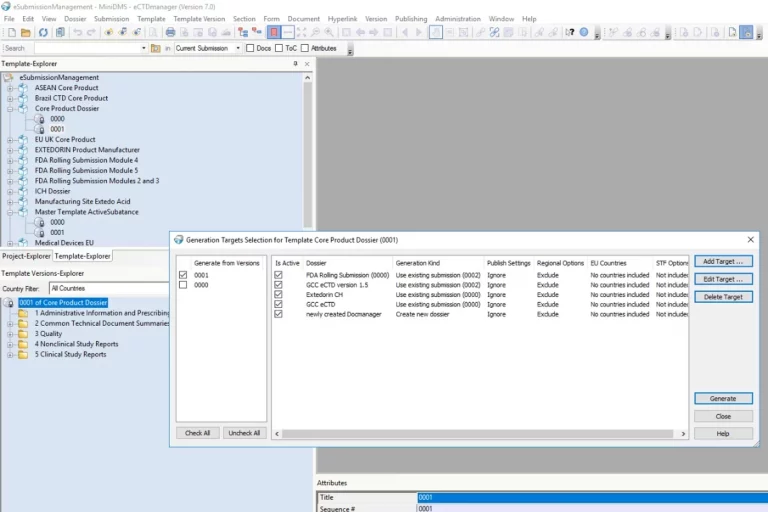
From authoring and publishing to ongoing management, eCTDmanager streamlines end-to-end regulatory submission processes, enabling organizations to scale their operations and generate error-free, compliant submissions.
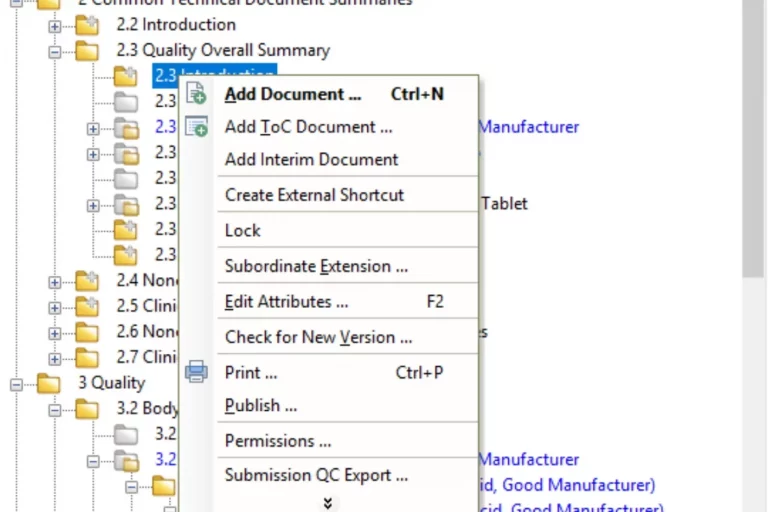
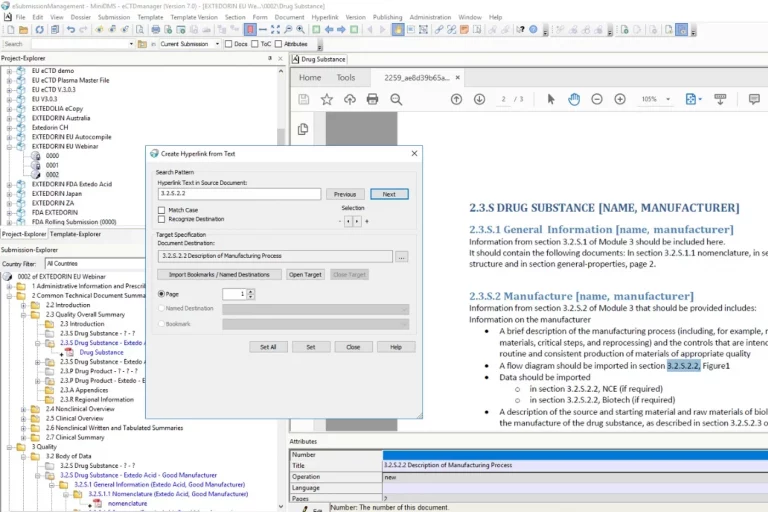
With eCTDmanager, you are able to build, view, validate and publish compliant submissions based on eCTD, NeeS, ACTD, eCopy, IMPD, PIP, VNeeS, DMF, ASMF, Clinical Trial Applications and other regional formats. eCTDmanager significantly improves the quality and consistency of your submissions, with a powerful hyperlinking and bookmarking engine that allows the detection, notification and correction of broken links. For FDA electronic submissions, eCTDmanager provides sophisticated Structured Product Labelling (SPL) capabilities that also enable data entry and maintenance of product information.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
An integrated solution that simplifies your lifecycle management
eCTDmanager goes beyond basic eCTD submissions software. It provides you with a complete regulatory dossier assembly environment that enables your organization to operate in a compliant manner within a heavily regulated environment. Its intuitive interface enables you to easily handle electronic submissions without prior knowledge of XML technology, and its unique visual aids provide context, ensuring simplified completion and unprecedented accuracy. The in-build technical validation ensures your submission is valid according to the latest validation criteria provided by the authorities.
About the company
When life science organizations release a new drug or medicinal product into the market, they are legally bound to adhere to regulatory submission and patient safety standards. These requirements can be complicated, and compliance is almost always time-consuming and expensive.
EXTEDO makes pharmaceutical compliance an effortless process. We provide solutions and expert knowledge that help life science organizations worldwide to reduce the time and effort required to create and submit regulatory applications for medicinal products and maintain them throughout their lifecycle.