Product Overview
The efficient production of compliant regulatory submissions is the goal of every regulatory operation. Having a single publishing solution that can effectively produce a variety of submission output formats provides a distinct advantage. This advantage includes terms of flexibility, training, and total cost of ownership.
Ennov Dossier is a complete and scalable dossier management and submission publishing solution. It is suitable for regulatory operations of all sizes. Ennov Dossier produces output that is compliant with all current health authority requirements:
- CTD (Common Technical Document) for paper publications
- eCTD and NeeS (Non-eCTD electronic submission) for electronic publications
- vNeeS for the veterinary sector
- eCopy for medical devices
Ennov Dossier is comprehensive and meets the most demanding requirements for regulatory submissions while still being intuitive and easy to use.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
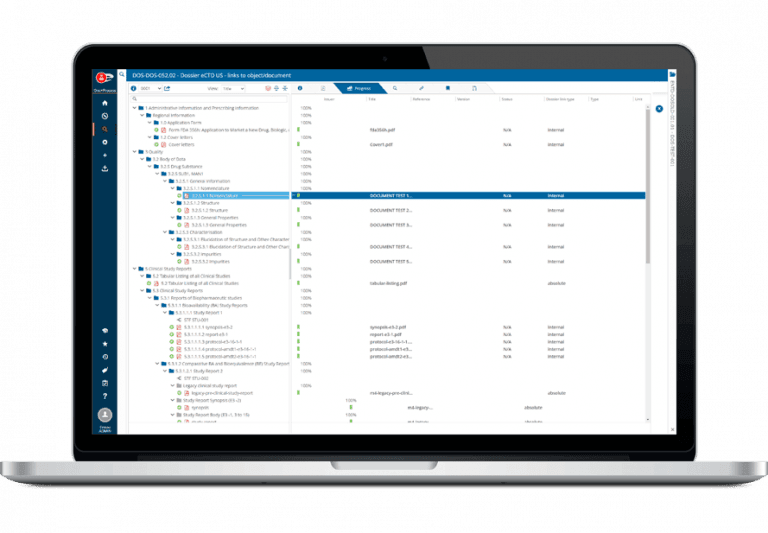
Ennov Dossier provides the ability to build, manage, publish, validate and archive regulatory dossiers using the native capabilities found within Ennov Doc.
This eliminates the fragmented and inefficient processes of locating, copying and uploading the documents that you need for your regulatory submissions – providing a harmonized and seamless dossier publishing solution.
A simple drag-and-drop interface allows publishers to link documents into submission assemblies quickly and easily.
Ennov Dossier, when combined with Ennov Doc, provides the ability to manage your regulatory dossiers using all of the robust functionality of our comprehensive EDMS.
Automated dossier life cycles, workflows and notifications eliminate fragmented manual processes and increase productivity.
Ennov’s unique metadata-based navigation also helps improve productivity by allowing users to quickly locate dossiers using their properties rather than a storage location in a filing hierarchy.
About the company
Headquartered in Paris, with offices in the US, UK, and Asia, Ennov provides the most original, comprehensive and cost-effective suite of software solutions for the Life Sciences industry. From leading pharmaceutical companies to emerging biotechs, we proudly serve over 250 companies and 250,000 users around the world.
For more than 20 years, we have been developing innovative, powerful and easy-to-use software for regulated content, data and process management. Our solutions are designed and built to support the entire Life Sciences R&D continuum including Clinical, Regulatory, Quality, Pharmacovigilance and Commercial. Ennov is ISO9001:2015 certified for all software products and processes and we boast a 100% success rate in customer audits.
Our solutions enjoy extremely high user adoption rates, thanks to our intuitive user interfaces and commitment to building solutions that work the way our users work. We also have very high customer satisfaction ratings with an on-time project delivery rate of 98.5% and an annual maintenance renewal rate of 96%.