Product Overview
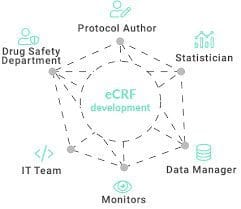
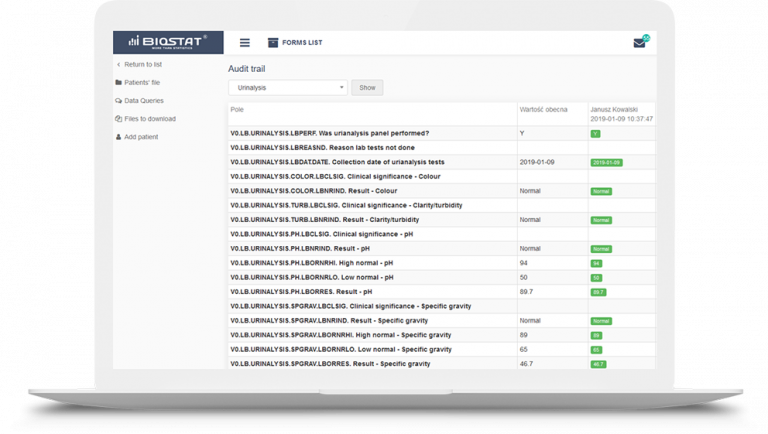
The clinical data management system – eCRF.bizTM will allow you to easily and securely collect data, while a friendly interface will be appreciated by your Investigators. The eCRF.bizTM is an integrated research platform that completely eliminates the need for paper clinical observational questionnaires, and lets us comprehensively manage entire studies. Electronic Case Report Forms (eCRFs) allow to obtain results much faster, and consequently allow for immediate review and analysis of data at each stage of the study.
Our clinical trial software – eCRF.BizTM system is used by a wide range of professionals and research centers, whose comments and suggestions provide valuable tips during constant development, resulting in continuous improvement. By consulting with our customers, we can design and implement the best possible solutions for specific monocenter or multicenter studies, tailored specifically to your needs and suggestions from your recognised partners.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Use our system in the following commercial and non-commercial studies:
- Drug clinical trials
- Medical device clinical trials
- Cosmetic and dermocosmetic clinical trials
- Bioequivalence studies
- Investigator-Initiated Studies
Our highly-qualified team have experience in development eCRF in the therapeutic areas like oncology, cardiology, alergology, dermatology, gastroenterology, psychiatry, ophtamology, neurology, etc.
About the company
BioStat is a R&D Center that specialises in Statistics, CRO, and Data Management in clinical trials. We also conduct Market Research projects. We provide essential research and consulting experience to experts in medicine, marketing & sales. Our team consists of qualified biostatisticians, statisticians and data managers with immense experience in research & business-oriented projects. We successfully cooperate with a number of pharmaceutical companies, marketing departments and scientific research centers.