Product Overview
JMP Clinical offers tools to make it easy for key participants in the trial review process to explore trends and outliers, detect hidden data, identify safety and efficacy issues, and perform medical monitoring, data integrity validation, and statistical analyses.
Clinical data scientists and medical monitors
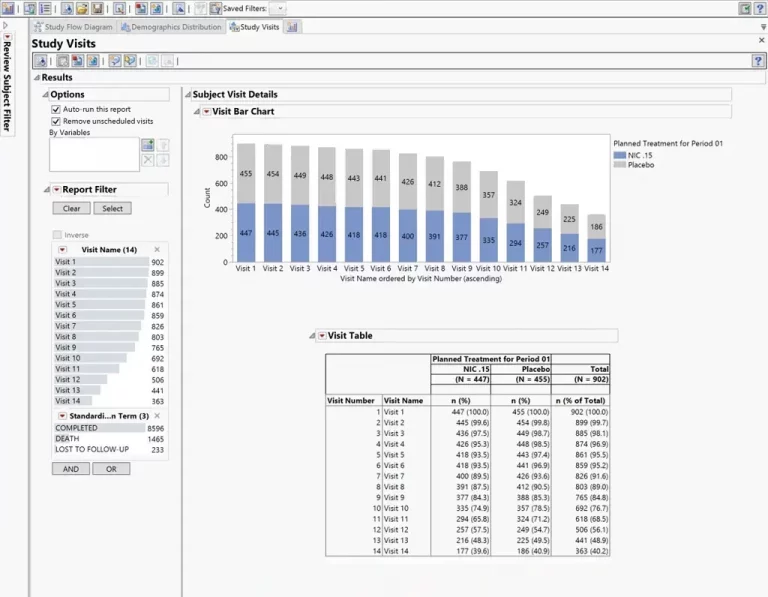
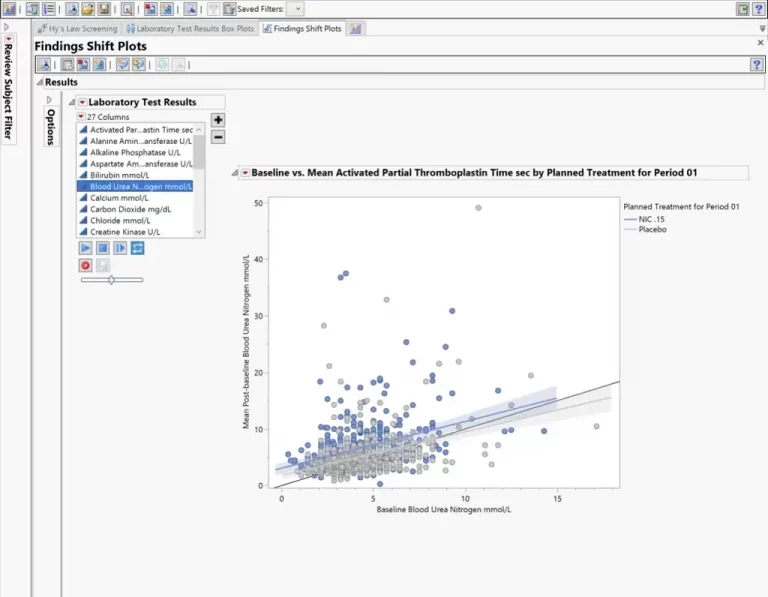
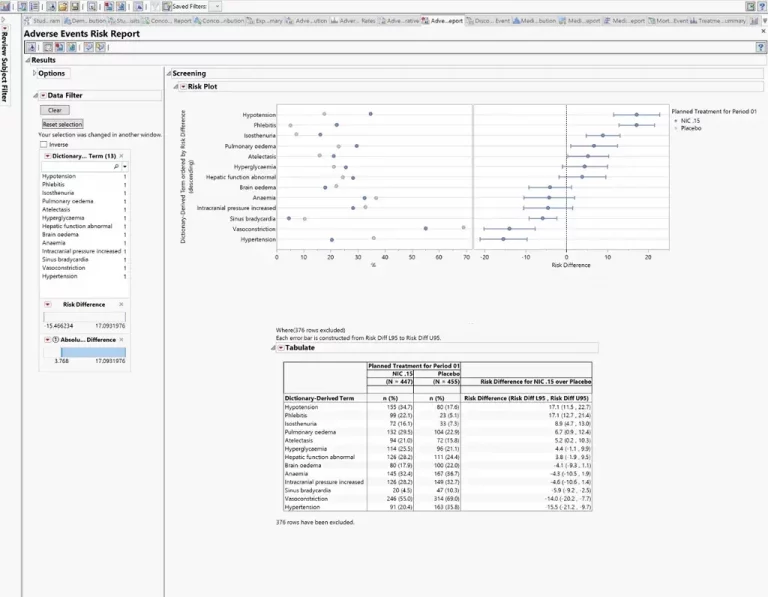
Summary dashboards in JMP Clinical enable medical reviewers to evaluate safety and efficacy issues with the click of a button. Create interactive reports of adverse events, concomitant medications, labs, and vital signs; drill down to customized patient profiles and patient narratives.
Medical writers
Producing adverse event narratives for clinical study reports (CSRs) can be a painstaking, time-consuming process with significant consequences for inaccuracies or delays. With JMP Clinical, medical writers can automate patient profiles and patient narratives to reduce the time and complexity of creating output for review and submission to regulatory agencies.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Clinical operations
The goal of clinical operations is to mitigate data quality risks that could hinder a regulatory submission or drug approval. Risk-based monitoring tools in JMP Clinical help you identify data anomalies at the vendor, monitor, site, and country level to determine the factors responsible for lapses in safety or data quality.
About the company
The JMP story goes back to 1989 when John Sall decided to combine statistical analysis capabilities with graphical visualizations to animate and visualize data. For more than 35 years now, John Sall has led JMP R&D, making each version of JMP more visual, more interactive, and more practical to help users understand their data. What started as a passion project has grown by leaps and bounds. It’s now a family of statistical software products designed with scientists and engineers in mind and used worldwide in nearly every industry.