Product Overview
Ripple can be considered a front-end to a CTMS in terms of purpose, design, and functionality. Most CTMSs provide CROs and sponsors with compliance tools to manage critical information from site-level teams, such as basic enrollment, billing, budgeting, and consenting information.
Ripple, in contrast, was built specifically for the study team to manage pre- and post-enrollment participant related processes, which are often outside the scope of a CTMS. Not surprisingly, many of our customers use a CTMS for compliance and billing purposes and Ripple for all other patient aspects of study management. Both are essential tools often used side by side for a more complete and efficient trial management experience.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
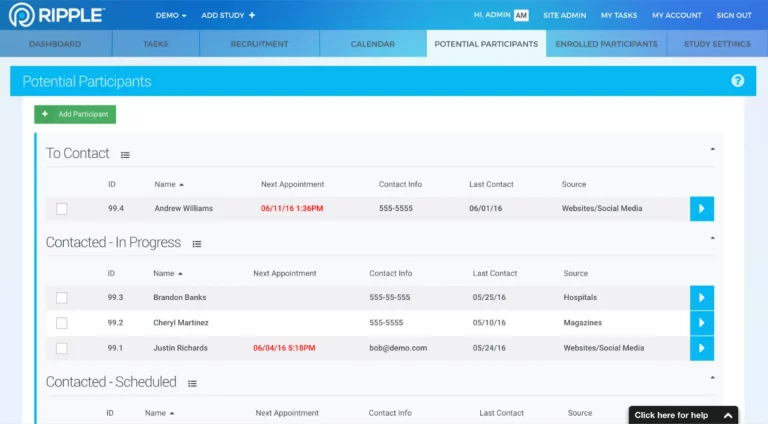
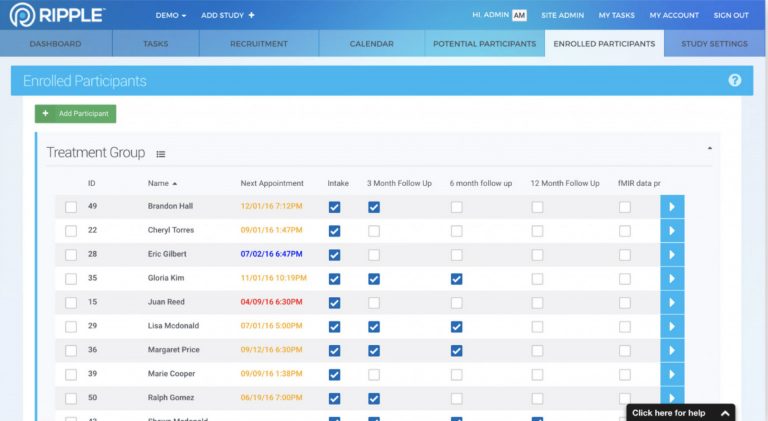
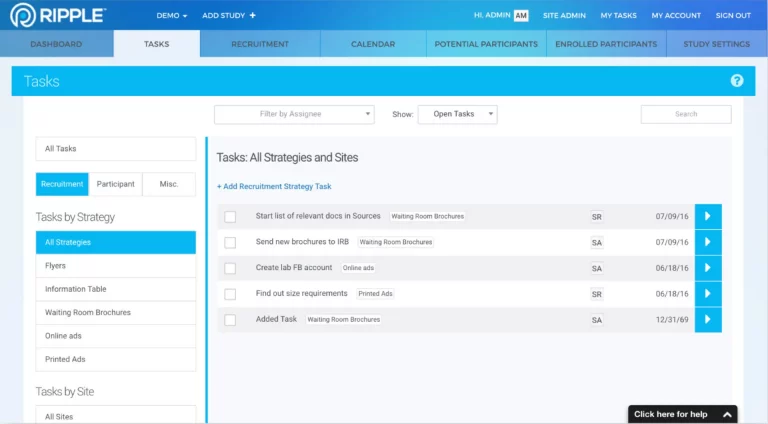
Although Ripple has many features found in a CTMS, Ripple works very differently. Ripple helps research teams at trial or study sites manage all aspects of the participant’s experience, including recruitment, randomization, activity tracking, appointment scheduling, and monitoring.
By enhancing a participant’s experience through better communication and effortless collaboration, your studies naturally become more patient-centered. Ripple helps your study team stay connected to the participants essential for your research.
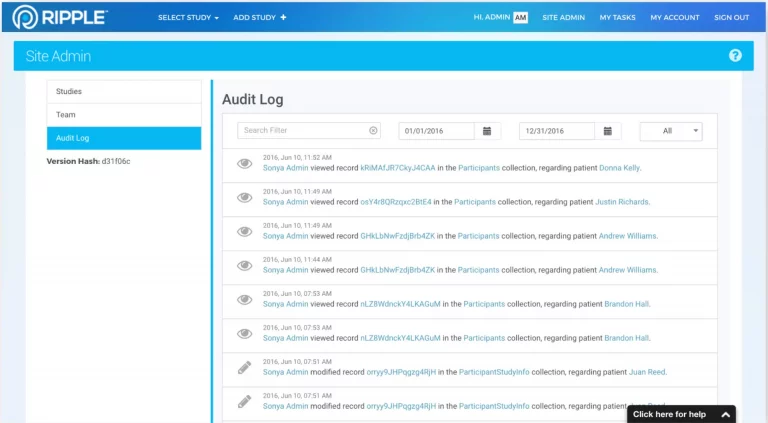
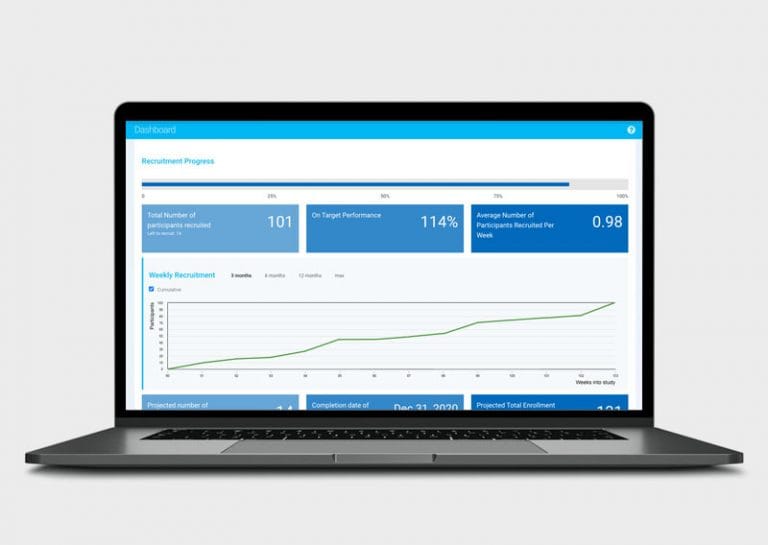
Ripple’s focus on the participant gives study teams, investigators, contract research organizations (CROs), and sponsors access to an incredibly rich set of participant-related actionable analytics, such as study progress, enrollment and completion projections, and site-level performance data for CROs and Sponsors.
About the company
Streamline Your Research with Ripple’s Participant Management Software
Ripple is an intuitive, web-based participant management software built specifically for clinical, translational, and social science research. Designed to support every phase of your study, Ripple helps research teams manage participants from recruitment through retention with ease and efficiency.
From the moment a participant expresses interest, itmakes it easy to track progress, schedule visits, send reminders, and capture study outcomes. Its user-friendly interface enhances communication between teams, improves coordination across departments, and allows for real-time data access. Whether you’re running a large clinical trial or a small social science project, it adapts to your needs and grows with your research.