Product Overview
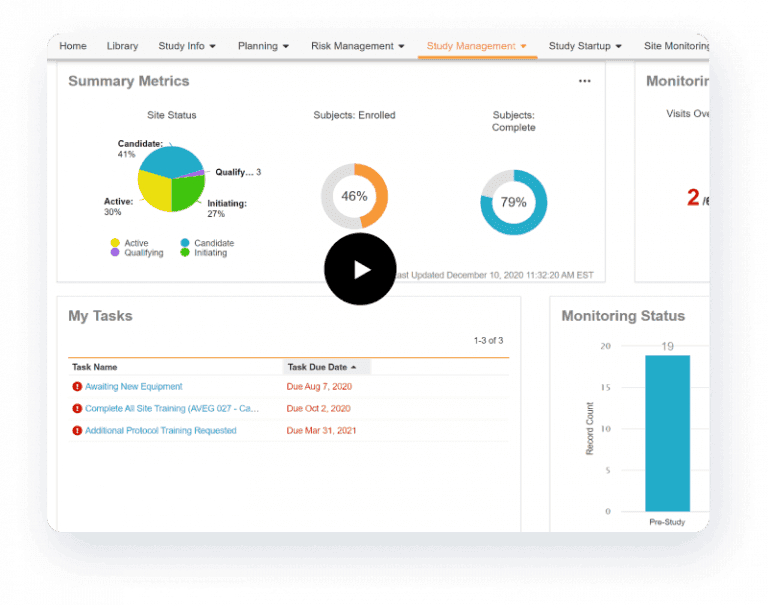
Veeva CTMS streamlines end-to-end study management and monitoring. Its dashboards and reports track key indicators such as enrollment and study milestones. The system automates monitoring-visit reports with dynamic question branching and files trip reports directly into Veeva eTMF. Study teams log issues and protocol deviations, then route them through resolution workflows to ensure timely closure. Through its tight connection with Veeva EDC, Veeva CTMS synchronizes enrollment, monitoring, payments, and one-click navigation to casebooks.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Faster, higher-quality trials
- Enhance productivity
Equip study teams with role-based dashboards and intuitive navigation. - Improve decision-making
Enable closed-loop issue management and strategic planning with a real-time view of trial status. - Speed trial execution
Proactively identify and manage risks to mitigate timeline slippages.
About the company
Our vision is to build the industry cloud for MedTech. More than just software. We are committed to helping the entire industry modernize to speed the total product lifecycle, bringing products to patients faster. We do this by partnering with the industry and MedTech companies to address the unique challenges we face.