Product Overview
Mural Health is the clinical research industry’s first participant management platform, designed to meet the needs of modern-day participants, help them enroll and remain in a trial, and reduce the administrative burden for sites.
Being part of a trial is often nuanced and time-consuming. Archaic travel, antiquated payment processes and card fees are not only frustrating, but can also chip away at the reimbursement funds participants are owed.
This isn’t what you want for your participants, but you haven’t had a better choice. If we have the technology to make things better, we should. So we are.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
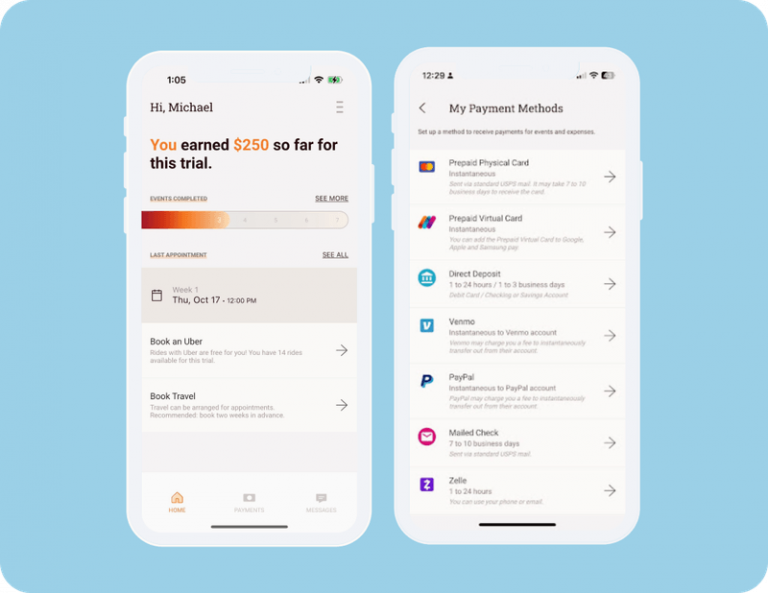
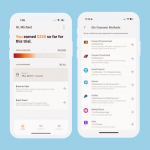
- Get paid however they want (with no hidden fees, ever).
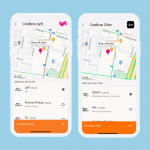
Participants choose from a growing list of payment options, including PayPal, Venmo, Zelle, direct-to-account, and the industry's first and only fee-free prepaid card. - Travel with no out-of-pocket expense.
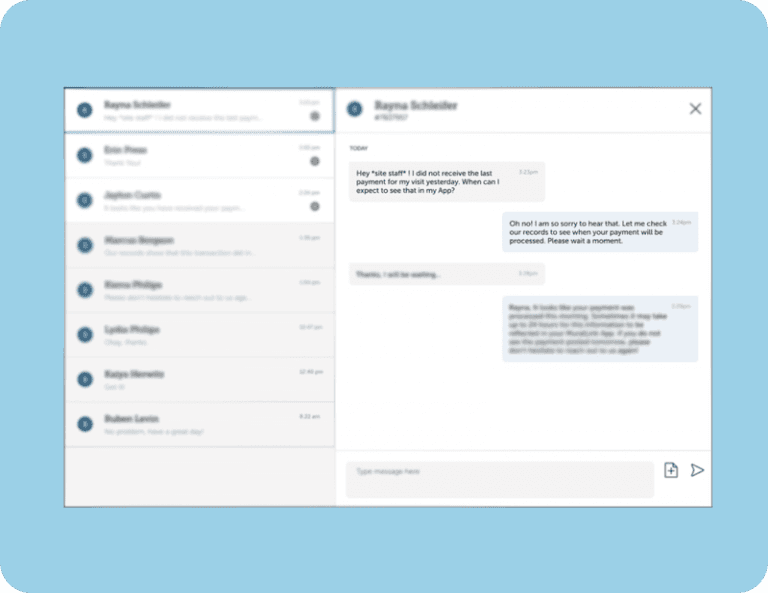
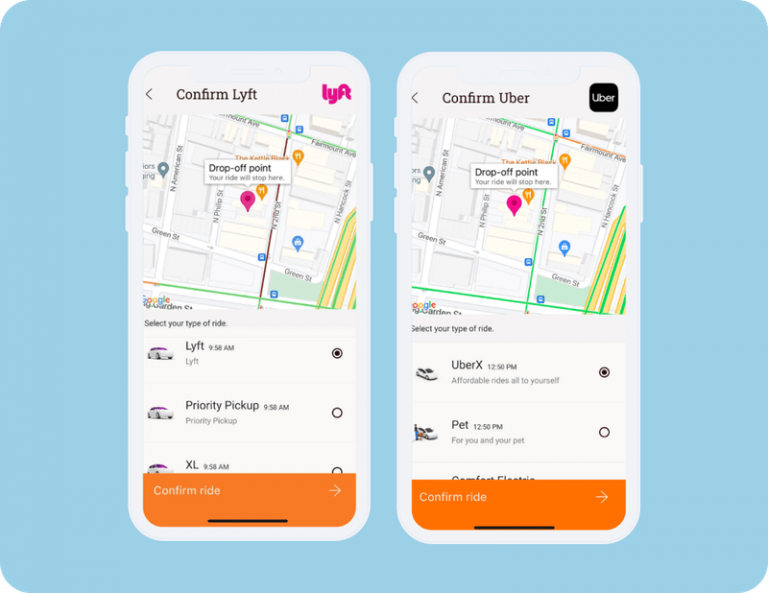
Participants can book their own transport via rideshare services right in the app, removing the need for cumbersome staff coordination and reimbursement. - Communicate and provide feedback easily.
With push notifications, in-app chat, and calendar syncing, participants always know what’s coming up next, how to prepare, and where to turn for help.
About the company
Clinical trials are designed to collect, organize and analyze participant data, in a way that is as efficient as possible. The aggregation of diverse human experience, across the world, is the underlying source of the data sets that drives progress in clinical R&D.
But a clinical trial cannot run successfully if we don’t take special care of the patients (and their caregivers) that make the data collection possible.
Being a participant in a clinical trial is not easy. Financial barriers and transportation challenges are just a few reasons, among others, that leave patients marginalized and feeling disconnected from the trial. These types of issues don’t only hurt participants, but often result in higher dropout rates and, consequently, impact the quality of data and ability to complete a trial.