Product Overview
TrialPal is a comprehensive electronic patient-reported outcomes (ePRO) and electronic clinical outcome assessment (eCOA) solution tailored for vaccine and decentralized clinical trials. It offers a unified smartphone, PWA, and web application designed to capture participant data—symptom diaries, adverse events, and more—with real-time alerts and offline capabilities
Rapid Study Setup
Create protocol-specific eDiaries, forms, and surveillance tools in under two hours—IRB-ready within 48 hours
eDiary & Symptom Reporting
Daily or multiple-daily entries enabled. Includes digital grading of symptom intensity, real-time AE/SAE alerts routed instantly to sites and stakeholders
Multi-Platform & Offline-First
Whether via native app, PWA, or web, participants can submit data offline, with automatic sync when
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Notifications & Reminders
Highly configurable push notifications, SMS, and emails to boost adherence within protocol-defined windows .
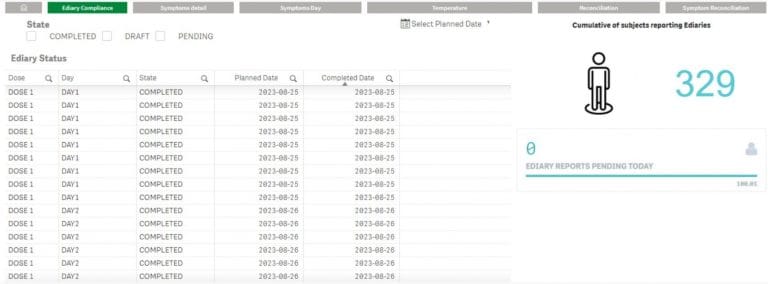
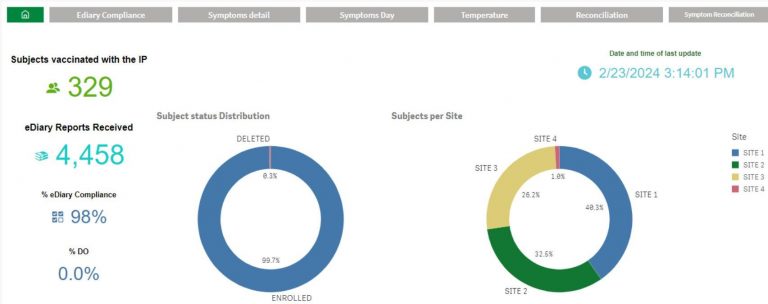
Site Console & Analytics
TrialPal Site offers a management dashboard for data entry status, eDiary reconciliation, audit trails, and downloadable PDF patient cards
Modular Expansion
Optional add-ons include Chat, eConsent (AI-powered multimedia, consent quizzes, e-signatures), Telehealth (beta), with an on/off architecture for hybrid or DCT setups
About the company
Founded in 2009, Integra IT is a company that uses innovation in technology and methods to facilitate access and surveillance of patients’ information in clinical trials, epidemiological and other Life Sciences related projects, in a time-reliable manner while improves clinical trial management and processes to streamline results. We offer a great portfolio of 100% cloud solutions, compliant with the highest security standards and guidelines such as the FDA 21 CRF part 11, with the aim of making clinical trials more efficient and effective.