Product Overview
Paper-based solutions and general-purpose clinical data collection systems are costly and clunky, slowing you down, and hindering your best work. Our modern eCRF is built for the unique needs of your device, and empowers you to start your clinical data collection the right way. Get started in no time and ensure peace of mind with our pre-validated software, regulatory templates, and user-friendly study builder.
Standardize Your Data Collection Activities
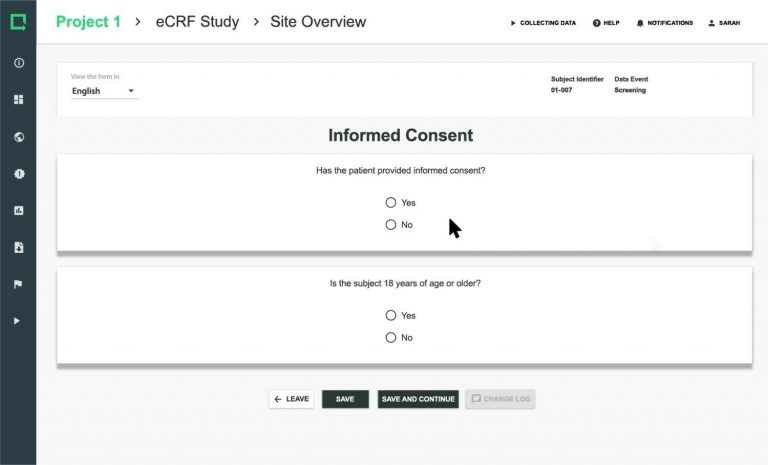
Greenlight Guru Clinical eCRF includes 17 different ready-to-use, pre-validated question types. Use these to standardize your data collection and avoid spending time validating your question design.
Build and Scale Studies Efficiently
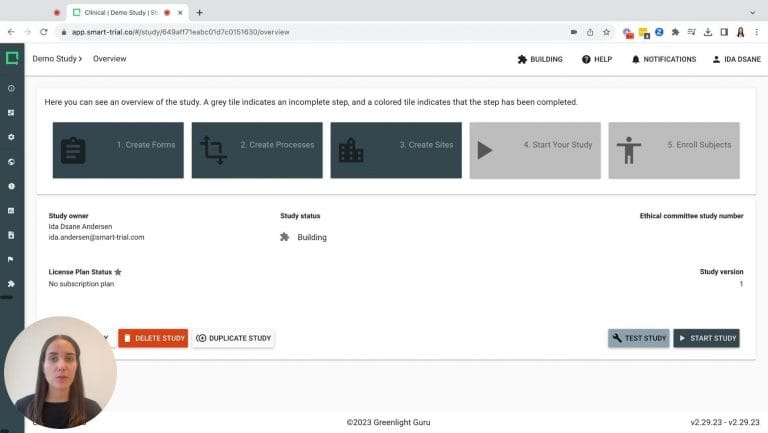
Build advanced eCRFs in minutes with our best-in-class study builder. Clone, reuse, and improve existing forms to streamline your study design and avoid setting up the same eCRFs again and again.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Simplify Clinical Data Compliance
Make clinical data compliance easy with built-in GCP compliant data monitoring and validation, regulatory compliance templates, and ISO 14155:2020 pre-validation.
See Your Data Your Way
Stay on top of your clinical trial progress at all times with real-time data monitoring and all your clinical data in one place or plugged into other business systems with best-in-class API access.
About the company
In 2006, Jon Speer, a medical device engineer turned consultant, had the initial idea for Greenlight Guru, when he made a simple observation: paper-based quality management systems (QMS) are painful, risky, and inefficient. At the time, commercial QMS solutions had been available for nearly two decades, but only 30% of MedTech companies were actually using them. This observation led Jon and his co-founder, David DeRam, to develop Greenlight Guru. Today, with our powerful, intuitive solution, MedTech companies across the world can bring higher quality, life-changing products to market faster, more efficiently, and with less risk.