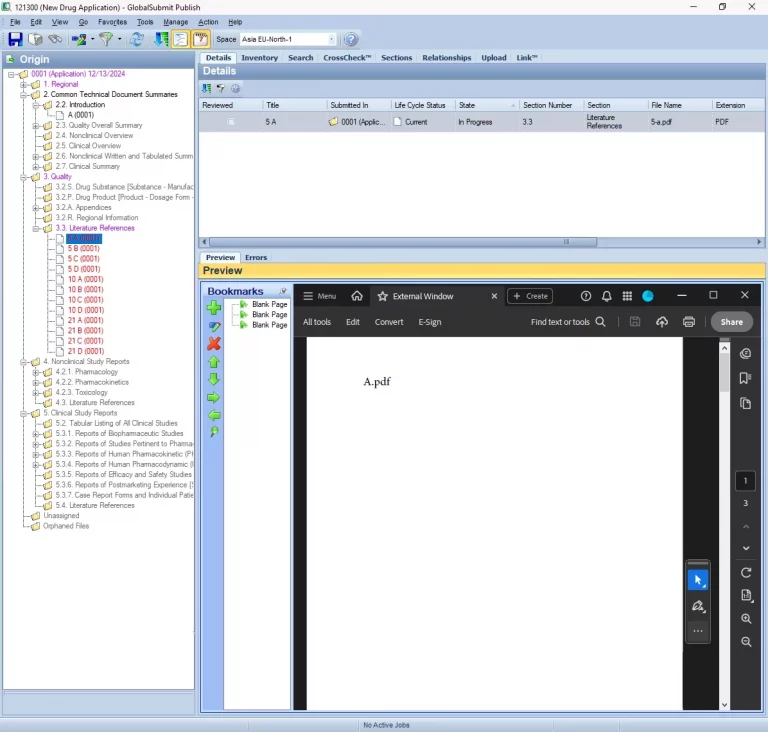
Product Overview
Navigating the complexities of electronic Common Technical Document (eCTD) submissions requires precision,efficiency, and collaboration. Certara GlobalSubmit eCTD Software empowers regulatory professionals to publish, validate, and review submissions seamlessly, as a team. By ensuring compliance with global regulatory standards, this advanced software eliminates risks and accelerates your journey to market.
- Comprehensive Publishing Tools: Simplify the creation of submission-ready documents with intuitive tools.
- Robust Validation Engine: Identify and resolve compliance issues before submission, reducing rejection risks.
- Streamlined Review Process: Conduct in-depth reviews with advanced navigation and annotation features.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
GlobalSubmit eCTD Software revolutionizes the way life sciences organizations manage regulatory submissions. Designed with a focus on speed, accuracy, and compliance, it provides a seamless platform to support your entire submission lifecycle. Whether preparing for a new drug application or updating an existing submission, the software ensures a smooth and efficient process.
About the company
We transform drug development for the benefit of humans. Our commitment is to empower innovators, researchers, and regulatory experts with software and service solutions tailored exactly to meet their needs, fostering success across the entire drug development lifecycle.
Certara is dedicated to transforming drug discovery and development for good. We harness the power of biosimulation, advanced analytics, scientific, strategic and regulatory expertise to create a future where treatments reach patients faster and more efficiently.