Product Overview
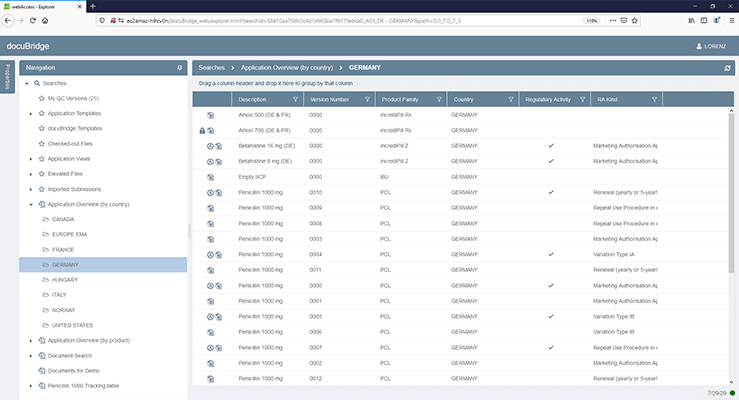
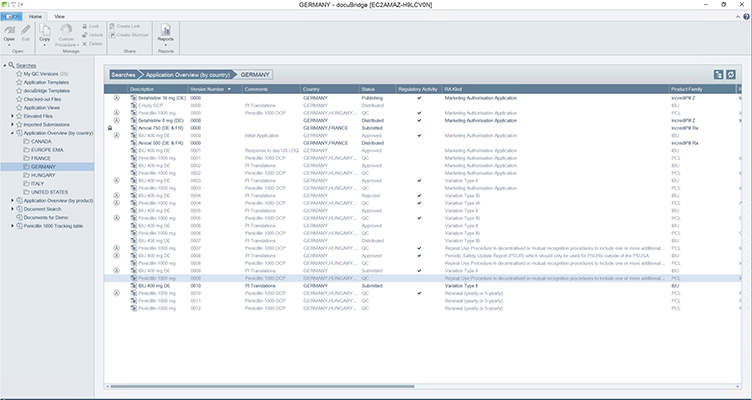
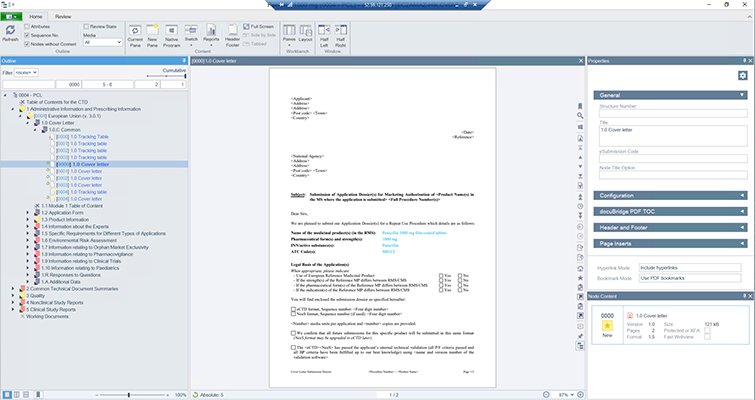
DocuBridge is an advanced electronic submission management and regulatory content management system for compiling, publishing, importing, and reviewing. As an eCTD publishing tool, it is also useful for other regulatory submission formats, including (V)NeeS, HTML, PDF and paper. Producing regulatory submissions has never been easier!
- Multi-format publishing: Publish multiple output formats from a single sequence: eCTD, NeeS, HTML, PDF, paper and more
- Team collaboration: Work as a team on a single regulatory submission sequence with no conflicts
- Ad-hoc specification updates: Receive regulatory submission specification updates automatically without reinstalling the software
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
- Interoperability
Work flawlessly with other LORENZ solutions and third-party software - Lifecycle management
Show current and revised versions of sequences, and which changes have been made. Organize sequences by regulatory activities - Flexible content reuse
Replicate the content files, hyperlinks, and node properties set in a source sequence to a lifecycle sequence in target applications
About the company
LORENZ’ tried and tested portfolio offers Product Registration/IDMP, Submission Assembly, Validation and Management, Publishing/eCTD, Regulatory Planning and Tracking products and related services. Interoperability between LORENZ products and third party solutions, as well as the ability to automate processes, allow LORENZ customers to enhance operational efficiencies.