Product Overview
Mono eCTD Office is a suite of integrated software products for the creation, validation, publishing, viewing and manipulation of regulatory documentation for electronic submissions by pharmaceutical companies to regulatory authorities.
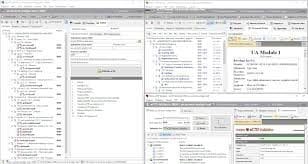
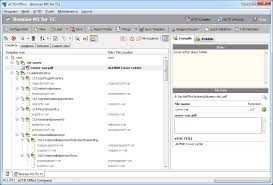
- Compile: Prepare you submission documents and fill in the provided regional-aware approved template. Add documents manually or import/merge an existing eCTD/NeeS.
- Behind the scenes: easily split and merge your PDF documents, convert MS Word DOC to PDF, define document Titles, attributes, granularity, envelope values
- Validate: Built in validation supporting regional rules ensures no invalid submissions can be generated.
- Publish: Fire-and-forget. No need to know anything about eCTD XMLs and other IT-related eCTD/NeeS aspects. NeeS TOC files auto-generated and optimized.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
View: Understand how Authorities see your electronic submission. Reduce review times, increase response times to Agency requests, and ensure faster approvals for your products.
Manage Lifecylce: As easy as breathing. Understand and manage your eCTD products lifecycle as simple as "assign-operation-target".
About the company
We are Mono, a full-service software development company from Osijek, Croatia. Team of 150+ developers, business analysts, project managers, UX/UI designers & quality assurance specialists – building bespoke software solutions to meet the specific needs of your business.