Product Overview
One Unified Platform for Clinical Data Management
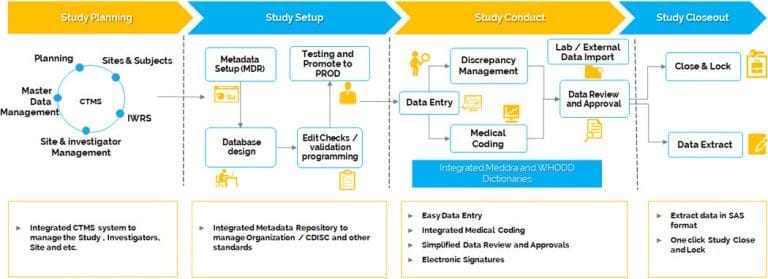
Clinevo’s Electronic Data Capture (EDC) / eCRF offers a powerful, all-in-one solution to manage every aspect of clinical data with ease and accuracy. From study planning and build to conduct, query resolution, medical coding, and data export, this platform supports your entire clinical data management workflow.
Designed for speed, accuracy, and compliance, Clinevo EDC helps sponsors, CROs, and research teams manage their studies more effectively—reducing timelines and improving data quality.
Built-In Quality Management System (QMS)
Clinevo EDC seamlessly integrates with Clinevo Quality Management System, allowing teams to manage CAPAs, change controls, deviations, audits, compliance documents, and training records from a single interface. This integration enhances visibility and simplifies oversight, ensuring consistent GxP compliance throughout the trial lifecycle.
Integrated Medical Coding
Medical coding is built directly into the EDC system. You can set up MedDRA and WHODrug dictionaries with ease and code clinical data in real time, reducing delays and improving data consistency across global trials.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Anytime, Anywhere & Any device
Clinevo Electronic Data Capture (EDC) / eCRF is the best EDC application which can be accessed with internet / intranet using IE, Chrome and Firefox browsers
Real time monitoring, Dashboards and reporting
Clinevo Electronic Data Capture (EDC) / eCRF provides powerful and actionable dashboards and reports for real time monitoring and tracking of study and site data and discrepancy / queries
Inbuilt Metadata (MDR) and Master Data Mgmt. (MDM)
Clinevo Electronic Data Capture (EDC) / eCRF is the best EDC that ships with inbuilt Metadata and Master data management modules to manage Study Global libraries, Sites, Investigators, Products and other master data in a centralized repository and provides a higher level of reusability of the information in different clinical trials.
Cost Effective & High Performing
Clinevo Electronic Data Capture (EDC) / eCRF is the best EDC system that comes with transparent optimized pricing.
Clinevo Electronic Data Capture (EDC) / eCRF is built on a Committed Infrastructure, hosting, training, business process and provides the best 24/7 Application support.
Dynamic workflows
Clinevo Electronic Data Capture (EDC) / eCRF is the best EDC that provides easily configurable workflows to meet any complex study requirements
About the company
Founded in 2016, Clinevo is a Software Development Company specialized in developing and implementing robust technology solutions for Life Sciences R&D. We help Pharma, Biotech and CROs in reducing their time and cost in Clinical trials by implementing innovative technologies that involves Data warehousing, Analytics, Collaboration, Automation, and Artificial Intelligence.
With our unique combination of domain experience & technology expertise, we are committed towards delivering the most efficient and the best practical end-to-end software solutions with HIPAA, GXP, CSV, 21 CFR Part 11 and other applicable regulatory guidelines. Our unmatchable domain experience and technology expertise enables us to deliver the best software solutions.