Product Overview
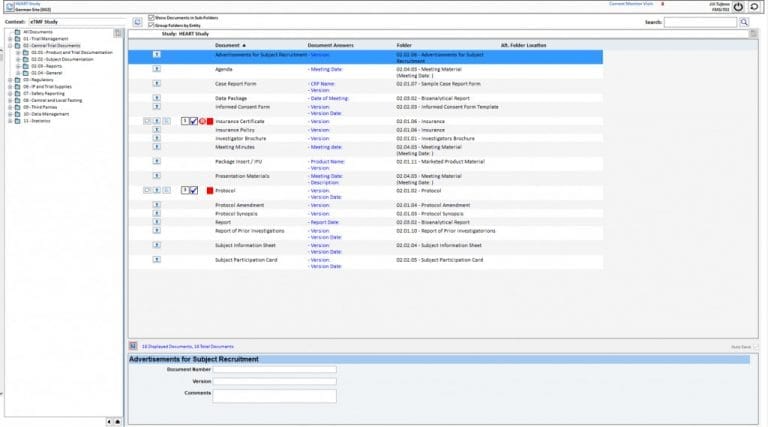
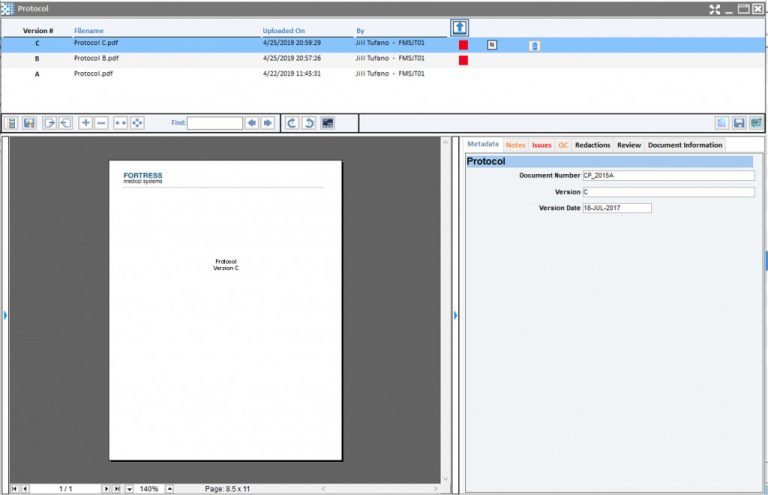
Stay in control of your trial with real-time access to study documents through the EvidentIQ eTMF. It’s easy to use and simple to manage. Build custom workflows to route documents automatically and eliminate time-consuming manual tasks. Since eTMF filing is built into our CTMS, your processes stay seamless and efficient.
Get instant oversight of documentation quality, completeness, and timeliness. Use built-in dashboards—or create your own—to track study health and monitor inspection readiness with ease.
Use EvidentIQ’s eTMF as a stand-alone solution or as part of our fully integrated platform. Either way, you’ll enjoy a single sign-on experience for all your clinical trial software needs.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
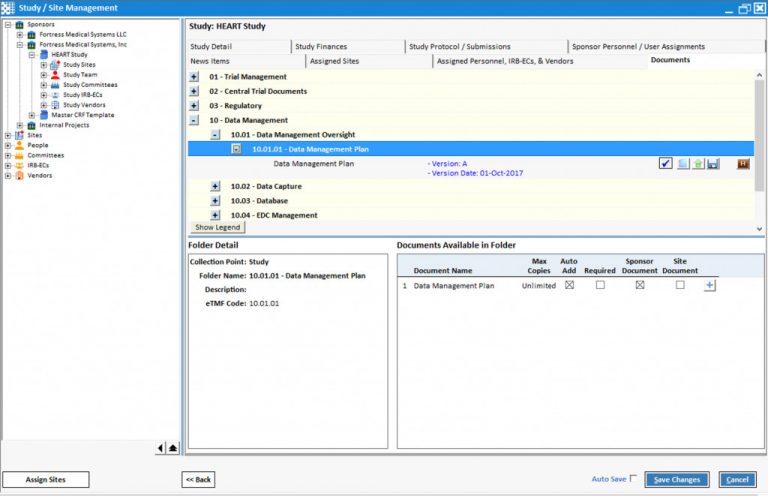
Flexible File Structure keeps you in control
You have complete control over the folder new person, new monitoring visit) structure, what documents you want to collect and what metadata you want to collect about them. Start from scratch or begin with our out-of-the box DIA reference model configuration (included at no
additional cost).
Intelligent Placeholders
Document Sets are created automatically based on events that occur (e.g. new site, You have complete control over the folder new person, new monitoring visit).
Integrated eISF
Provide your sites with access to the integrated eISF, reducing burden for your sites and your monitors while ensuring oversight of clinical trial documentation.
Cost Effective
Subscription based software with no per study fees. Use in conjunction with our CTMS for even more cost efficiencies
About the company
Leveraging more than 50 years of combined experience providing innovative and creative ways to conduct clinical trials. End-to-end eClinical Solution
Meeting increasing customer demand across clinical operations and clinical data management needs with a suite of applications within a single integrated cloud platform, including EDC, CTMS, eTMF, eCOA, coding, remote monitoring, RBM, RTSM, Payments and eFeasibility. Geographical Reach
In-country patient communities platform in North America and Western Europe (EU5) and ability to conduct studies at a broader scale (LatAM, Asia…). Regulatory expertise to support broader geographical studies.