Product Overview
Streamline clinical regulation and management activities within your organization; boosting productivity and eliminating human error.
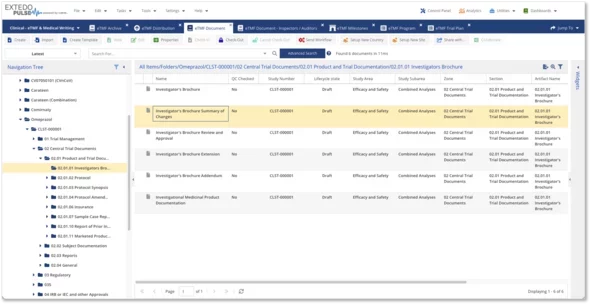
The eDOCSmanager Clinical Module enables you to use a template-based approach for simplifying document creation and generating eTMF structures on demand. Reduce data duplication and errors with dashboards and reporting based on metadata to manage all of your eTMF information. Now, you can rapidly create TMF files and manage the entire eTMF lifecycles from start to finish from one location.
Manage your eTMF data effortlessly
The Clinical Module gives you access to customized dashboards and reports based on metadata to help manage your eTMF information. With easy-to-use eTMF templates, drag-and-drop functionality, and complete integration with the other processes of your organization, it’s never been easier to create, manage and submit your eTMF files.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
A secure document management system for clinical trials
Poor information and document management practices during your clinical trials put the health of patients at risk.
eDOCSmanager provides a platform for managing clinical trial documentation in a controlled and automated manner; enabling you to minimize compliance risks and trust that the patient data you rely on is accurate and safe.
About the company
When life science organizations release a new drug or medicinal product into the market, they are legally bound to adhere to regulatory submission and patient safety standards. These requirements can be complicated, and compliance is almost always time-consuming and expensive.
EXTEDO makes pharmaceutical compliance an effortless process. We provide solutions and expert knowledge that help life science organizations worldwide to reduce the time and effort required to create and submit regulatory applications for medicinal products and maintain them throughout their lifecycle.