Product Overview
Montrium eTMF Connect: More Than Inspection-Ready
Montrium eTMF Connect doesn’t just prepare you for inspections—it transforms the entire inspection experience. Thanks to its intuitive interface, inspectors can be trained quickly, helping you save time and reduce stress during critical moments.
Smooth Collaboration, Global Access
- A centralized records center allows regulatory agencies to review documents seamlessly.
- Cloud-based and globally accessible, the platform removes location-related barriers.
- A specialized inspector portal ensures quick, easy access to essential files.
- Smart filters make it easy to find and navigate TMF content.
Stay in Control of Your Study
With eTMF Connect, you always have a clear view of your TMF activities. Because all study information and documents are stored in one place, you know exactly what steps to take next—without wasting time.
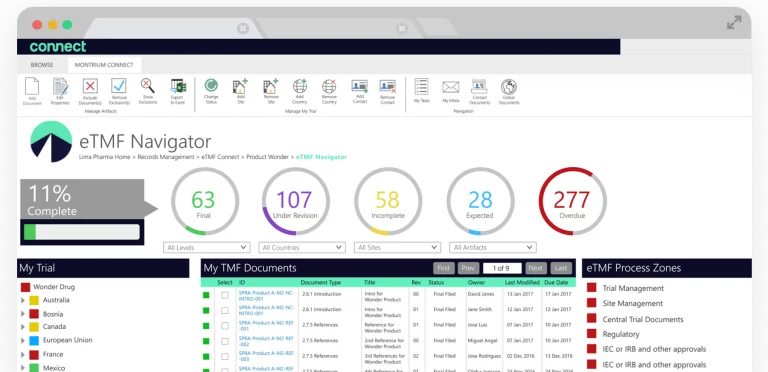
Get Real-Time Oversight
The built-in eTMF Navigator helps you instantly spot missing or expected documents. Once identified, you can drag and drop files directly onto placeholders, which updates your TMF completeness in real time.
Built for the Way You Work
eTMF Connect is centralized and unified, bringing all study stakeholders into one collaborative space. Because it adapts to your unique processes, it gives you both agility and clarity—ensuring smarter and more efficient workflows.
Automate the Tedious, Focus on What Matters
By automating tasks like drafting, reviewing, and approving documents, eTMF Connect gives you valuable time back. You can rely on the platform to handle repetitive work, so you can focus on driving your clinical trial forward.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Create better inspection experiences. Easy to use, easy to train
eTMF Connect goes beyond simply improving inspection readiness to improving the entire inspection experience. Its intuitive interface means that you’ll be able to train an inspector in the system in no time, saving you valuable hours and alleviating unnecessary stressors during an inspection.
Empower a remote workforce. Enable decentralized clinical trials
Never in history has it been more business critical to have tools that facilitate remote collaboration. With eTMF Connect, team members across the globe can securely access and contribute to the trial master file with ease, improving inspection readiness and regulatory compliance.
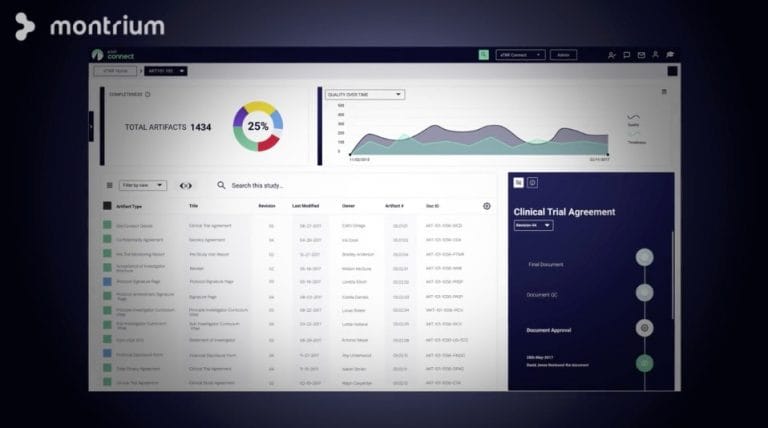
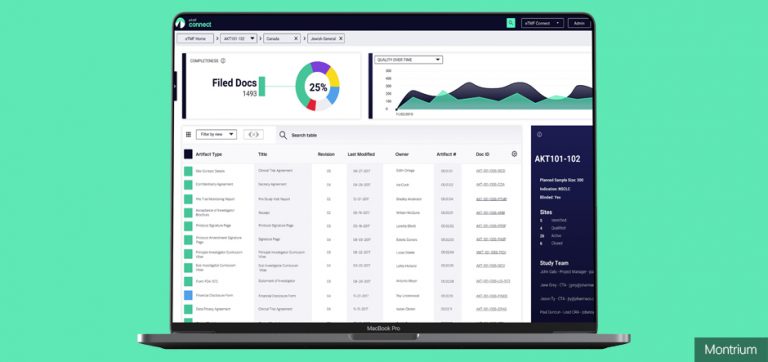
Get real-time study intelligence. Powerful reporting & dashboards
You can't fix an issue if you don’t know it exists. With eTMF Connect, you’ll be able to access powerful reporting features and real-time dashboards that allow you to detect problems before they grow into inspection findings.
About the company
Montrium is a global leader in cloud-based records, submissions, and quality management solutions and GxP consulting services for the life sciences. We help organizations implement and maintain technology to improve their business processes and increase compliance. Delivering our powerful content management solutions across the globe, we serve thousands of users in over 20 countries.
At Montrium we believe in providing the best possible products and services, and our ability to understand the unique needs of each customer is the reason why our customers continue to return to us every time. We work together as a team to always put our customers first, focusing on close collaboration between our clients and our team members. Our transparent approach towards any new opportunity has allowed us to create bonds of trust with some of the largest players in the life sciences industry.