Product Overview
Accelerate study start-up with proactive oversight
Fast and efficient site activation is key to bringing new treatments to patients. HORIZON, ICON’s advanced study start-up solution, simplifies the site activation process, giving you the visibility, control, and data insights to keep studies on track and meet ambitious timelines.
Features
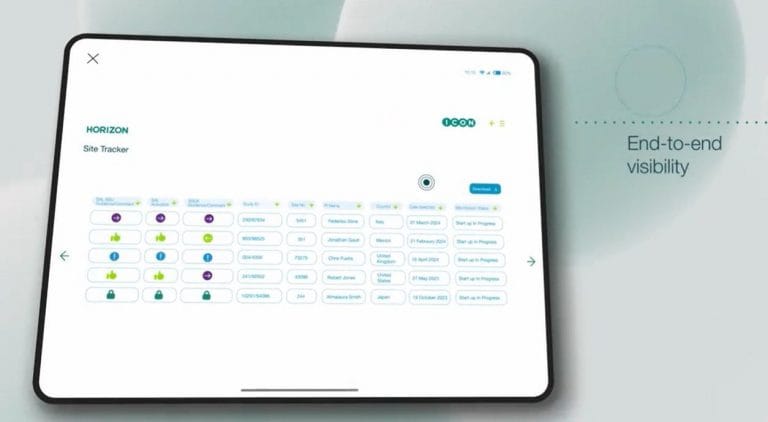
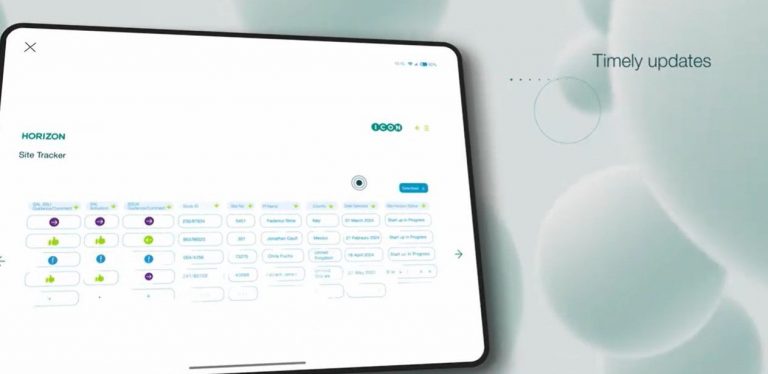
- Real-time centralised dashboard – HORIZON offers a complete, real-time view of study and site activation progress. Consolidating data from multiple systems, HORIZON’s centralised dashboard provides transparency into each site’s status, pending tasks, and key dates, for quick, informed decision-making. Horizon allows users to visualize the study activation plan, as well as how disruptions such as protocol amendments, will impact the planned activations by site.
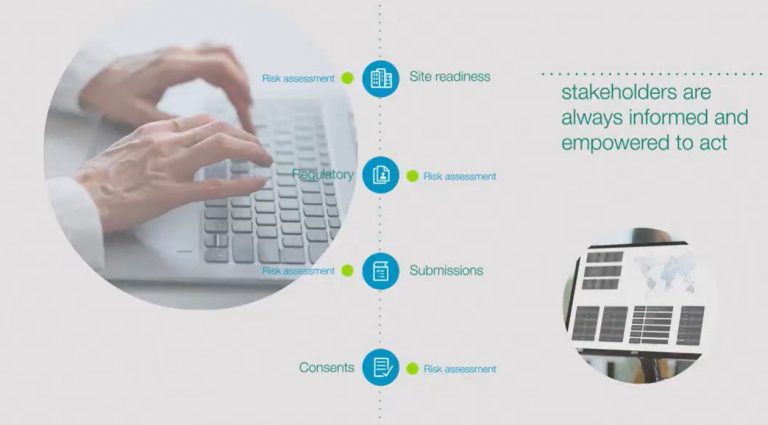
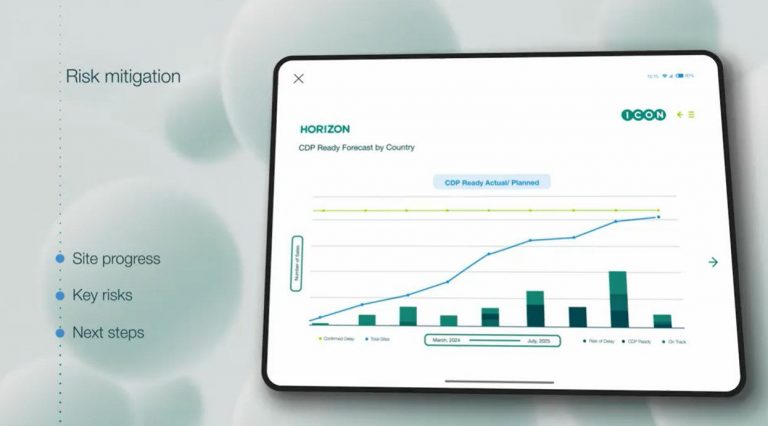
- Early risk detection and mitigation – Gain an edge with proactive risk management. HORIZON identifies potential delays to each task and site on a real-time basis, allowing you to minimise disruptions and address issues before they impact timelines.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
- Automated workflows and task prioritisation - Remove the burden of repetitive, manual tasks with automated workflows for contract reviews, informed consent approvals, and more. HORIZON’s task prioritisation features help you stay focused on high-impact activities, improving efficiency and accelerating study timelines.
- Seamless collaboration across teams – Enable seamless collaboration among study start-up, clinical teams, and sponsor stakeholders. With consistent access to real-time data, each team can confidently coordinate and execute on shared priorities.
- Predictive metrics for informed oversight – Get visibility into key metrics across sites, teams, and regions. By understanding performance in real time, you can anticipate next steps, compare site activations, and ensure alignment across all stakeholders for streamlined execution.
About the company
Since our foundation in Dublin, Ireland in 1990, our mission has been to help our clients to accelerate the development of drugs and devices that save lives and improve quality of life.
We are a global provider of consulting, and outsourced development and commercialisation services to pharmaceutical, biotechnology, medical device and government and public health organisations. We focus our innovation on the factors that are critical to our clients – reducing time to market, reducing cost and increasing quality – and our global team of experts has extensive experience in a broad range of therapeutic areas. ICON has been recognised as one of the world’s leading Contract Research Organisations through a number of high-profile industry awards.
ICON’s technology solutions are focused on the factors most critical to our customers. With expertise in technology harmonisation and systems integration, our innovative tools and platforms can be adopted smoothly, to enhance collaboration, increase transparency and drive productivity gain.