Product Overview
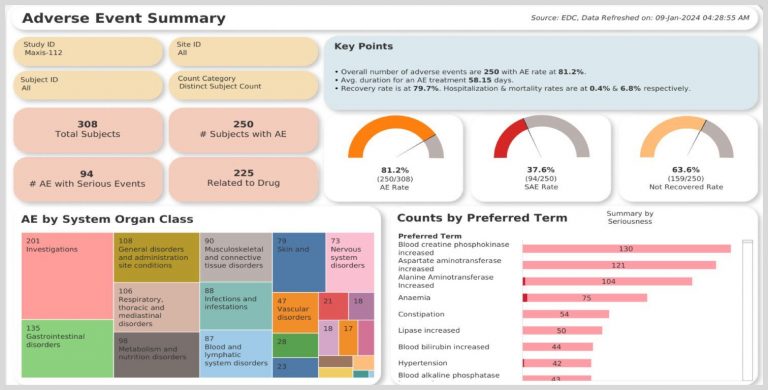
Get clinical insights that drive real results with unified clinical, operational, and real-world data. The Clinical Trial Oversight System (CTOS) allows you to expertly monitor and manage the entire clinical ecosystem.
Develop protocol strategies, monitor progress on decentralized clinical trials, and conduct data quality management at the micro and macro levels.
- Automated Processes: Integrate and automate the data-to-analytics process to mitigate risks, save time, and avoid expensive delays, errors, and bottlenecks.
- Continuous Oversight: Gain oversight at study and portfolio levels, standardizing data ingestion from disparate systems and sources for easier analysis and reporting.
- Patient Safety and Trial Integrity: Ensure patient safety and trial integrity with data-driven oversight, powered by a preconfigured library of hundreds of metrics for spot-on monitoring.
- Study Tracking: Keep studies on track with alerts, role-based controls, version controls, and interactive dashboards.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
- Real-Time Access: Get real-time access to a single-source-of-truth data repository to meet important milestones.
- Risk Indicator Detection: Monitor adverse events, screen failures, and query response rates to gauge site performance and detect concerns.
- KPI Optimization: Pinpoint and optimize key performance indicators at the crucial sites and CRO interfaces.
- Fraud Detection: Detect outliers early to safeguard your portfolio and ensure compliance.
About the company
Maxis AI provides data management, clinical operations, and decentralized trial tools. By giving you timely oversight into clinical, operational, and healthcare data, our source-agnostic solutions help you plan, run, and manage more cost-effective clinical trials.
Over 3,300 clinical trials to date have gained more efficient processes, more accurate data, and more comprehensive analyses by using our advanced platform and expert services.