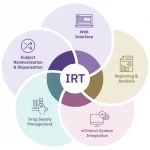
Product Overview
Managing clinical trial randomization and supply logistics can be challenging, especially with the complexities of modern clinical trials. Hybrid trial approaches, tight timelines, and the need for mid-study adjustments mean that sponsors and sites need precise and powerful systems to keep trials running efficiently and on-time.
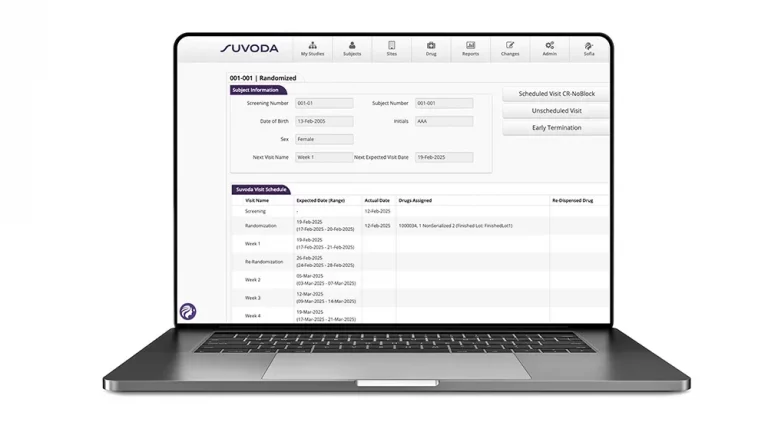
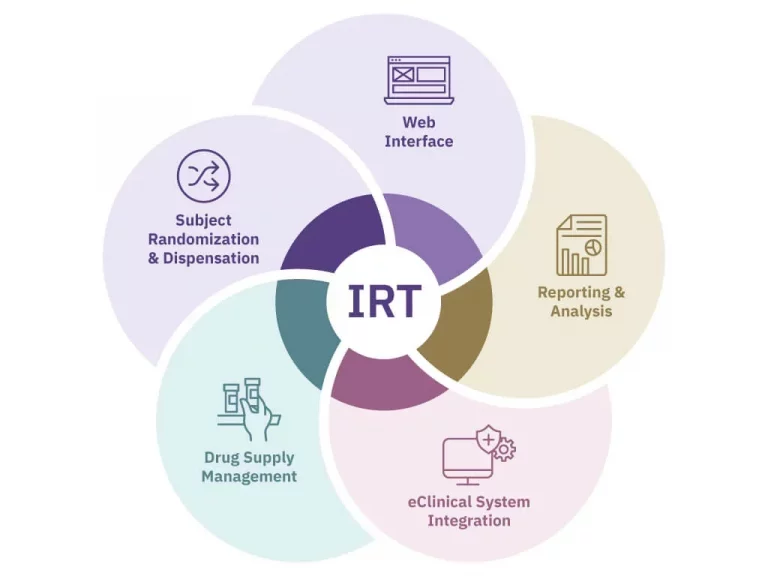
From initial setup to mid-study adjustments to study completion, Suvoda Interactive Response Technology (IRT) gets the right drug to the right patient at the right time, with advanced randomization and drug supply management capabilities that integrate seamlessly with existing clinical workflows and eClinical systems.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
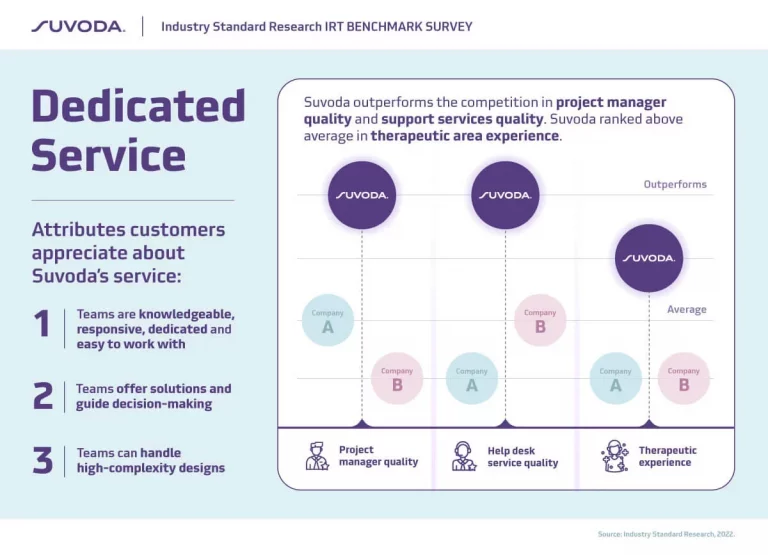
Suvoda IRT keeps trials running smoothly and supports sites, sponsors, and CROs to:
- Manage all clinical trial workflows efficiently, with data that is shared across systems
- Meet exact trial randomization and drug supply needs with extensive configurability and customizability
- More simply conduct patient visits and drug dispensation with an intuitive interface and user-centric design
- Access robust, real-time reporting for enhanced trial oversight and decision-making
About the company
Suvoda is a global clinical trial technology company focused on complex studies for life-saving therapies.
The Suvoda Platform lets you manage time-sensitive, high-impact trial activities with ease.
It combines eConsent, IRT, eCOA, and ePatient tools in one simple, purpose-built system.
Configure your trial tech to fit your exact needs.
Use fast integrations, tools that maintain standards, and a platform designed for quick deployment.
After launch, Suvoda improves the study experience for teams, sites, and patients.
Everyone stays engaged and can contribute with confidence.