Product Overview
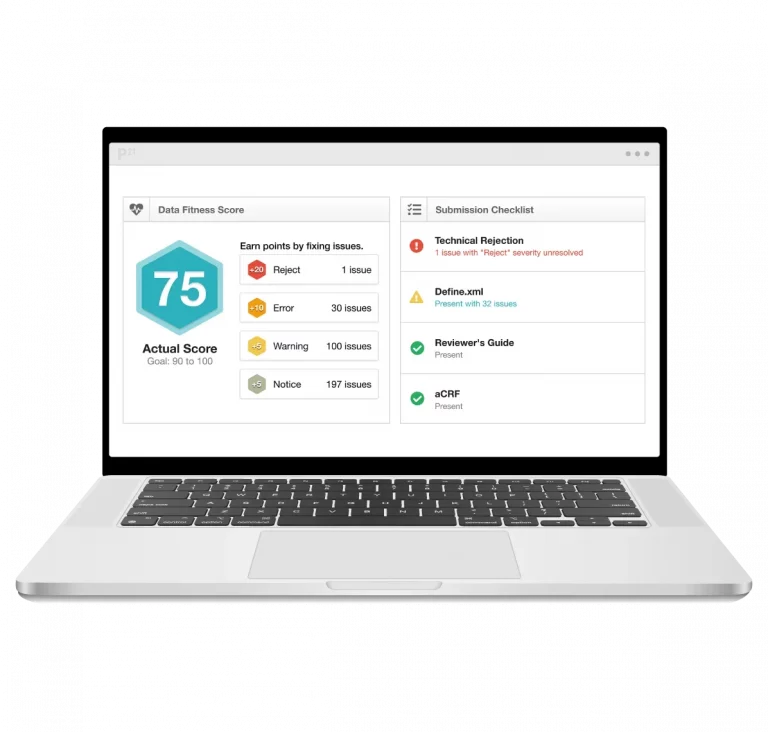
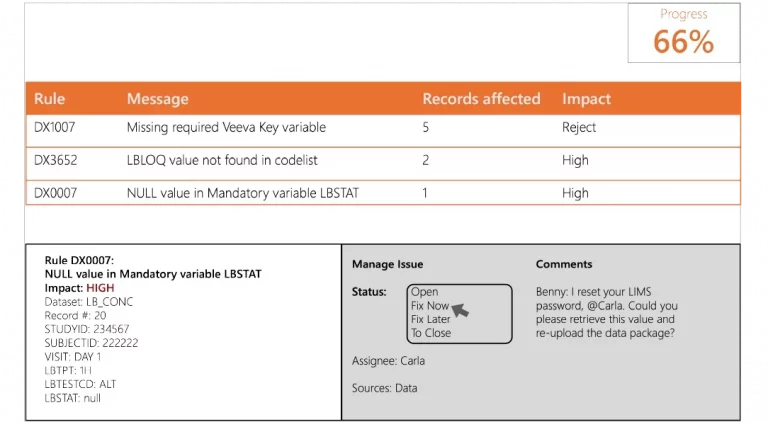
Pinnacle 21 clinical data standardization software empowers clinical teams to standardize, validate, and harmonize data effortlessly. With Certara Pinnacle 21 Regulatory, achieve flawless submissions and accelerate development with confidence.
- Manage and optimize your clinical trial data from the start.
- Shorten the time to analysis – and submission-ready CDISC datasets, Define XML files, and Study Data Reviewer’s Guides.
- Collaborate with internal stakeholders, CROs, vendors, developers and others in a central workspace.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
The Pinnacle 21 clinical data management software platform transforms clinical data into submission-ready deliverables – enhancing data quality across studies, and validating datasets against global regulatory standards.
By integrating metadata management and continuous data validation in a collaborative platform, Pinnacle 21 speeds timelines, reduces risk, and ensures data integrity throughout the clinical data flow.
About the company
We transform drug development for the benefit of humans. Our commitment is to empower innovators, researchers, and regulatory experts with software and service solutions tailored exactly to meet their needs, fostering success across the entire drug development lifecycle.
Certara is dedicated to transforming drug discovery and development for good. We harness the power of biosimulation, advanced analytics, scientific, strategic and regulatory expertise to create a future where treatments reach patients faster and more efficiently.