Product Overview
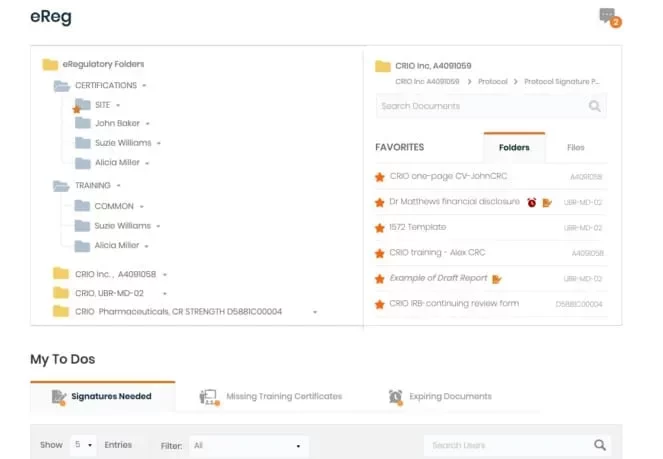
Instead of filing the same GCP, IATA and other common files multiple times, site users can file them once for the site or themselves, and then let CRIO file into each study automatically in Clinical Research IO eREG. Expiration alerts and versioning ensure users keep their credentials up to date.
Manage study ISF binders digitally
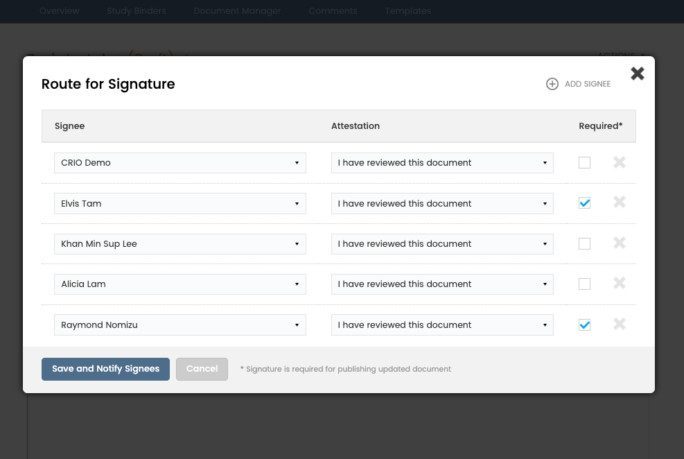
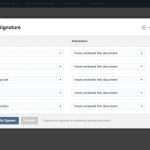
Upload, version, route, and e-sign regulatory documents. Configure study folders and save favorite folder structures as templates. Track expiring documents, bulk sign documents, and view/respond to CRA queries.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
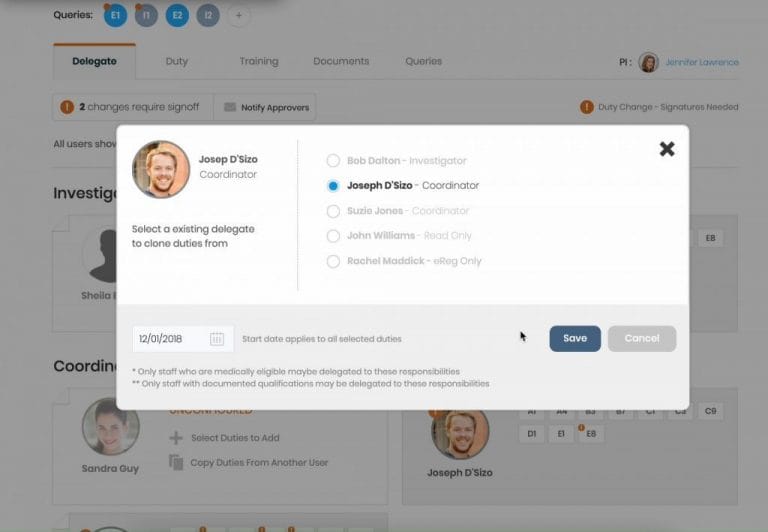
Digitize the Delegation Log
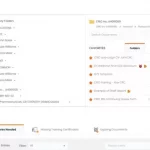
Study teams can build electronic delegation logs. Copy duty profiles from one user to the other. Easily keep track of requested and delegated duties. Each user can view their own personalized delegation log with one click. CRAs love our downloadable, print-friendly DOA log with legible audit trails!
About the company
Founded in 2015 by current CEO Raymond Nomizu and Phuc Truong, CRIO’s goal is to transform the clinical trial industry by reimagining workflows from a site-centric perspective. By automating protocols at the site level, CRIO’s technology builds quality at point of capture, unlocking significant downstream benefits for sites and sponsors alike.