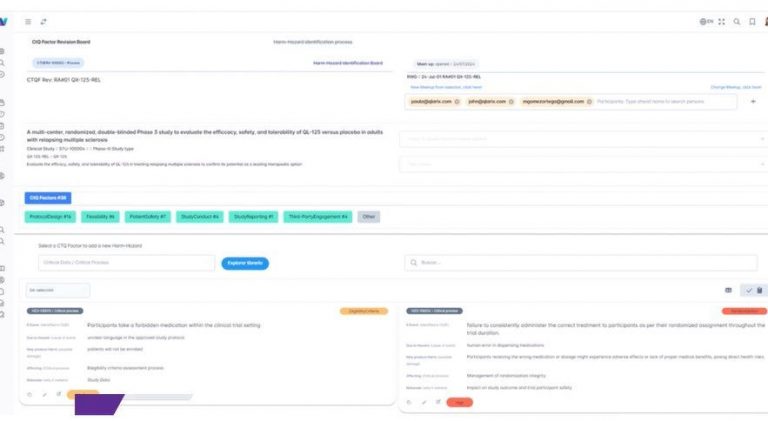
Product Overview
Partnering with CROs worldwide, WiseCLIN delivers innovative RBQM and oversight functionalities that align seamlessly with Sponsor expectations and regulatory requirements. Our intuitive tools and comprehensive training programs empower CRO teams to streamline trial management and enhance operational performance. By customizing our approach, we ensure both you and your Sponsors achieve superior trial outcomes and compliance excellence.
Recognizing the specialized needs of academic and independent research institutions, WiseCLIN offers customizable solutions that adapt to each project’s goals and complexity. Our platform supports data integrity and risk management, ensuring the credibility and reliability of your findings. With dedicated project management and long-term support, we help research entities advance research discoveries and achieve meaningful scientific impact.
Deployment
- Web based
- Cloud Hosted
Pricing
- Subscription
- License Fee
More Information
Designed with the challenges of medical device development in mind, WiseCLIN accelerates your path from concept to market. Our platform provides robust support for generating reliable clinical evidence, meeting stringent regulatory requirements, and adapting to the evolving medical device landscape. We help you maintain confidence and compliance while ensuring your devices reach patients efficiently and effectively.
About the company
At Qlarix, we empower organizations to embrace Risk-Based Quality Management (RBQM) through cutting-edge technological solutions designed to elevate the integrity and efficiency of clinical trials. Our solutions, such as WiseCLIN, merge research expertise with innovation to foster a robust risk management culture.
To leverage expertise, innovation, and technology to empower life sciences organizations with effective clinical trial oversight. We aim to foster a culture of robust risk management, ensuring the highest standards of quality and compliance while driving advancements that improve global healthcare outcomes.